Lecture 1: Introduction and ‘Atomos’
0) Orientation: How this course works and what to expect


The Properties of Matter (PoM) course, taught by Dr Ross Springell, an Associate Professor specialising in solid-state and condensed matter physics, focuses on many-particle systems such as gases, liquids, and solids, contrasting with the single-particle focus of classical mechanics. Dr Springell's research includes fundamental physics of heavy elements (actinides like uranium and thorium), nuclear fission fuels, nuclear waste materials, and tritium storage for fusion. Students are expected to maintain their own notes, as no pre-written lecture notes are provided; however, slides and handwritten derivations will be uploaded to Blackboard after each lecture. Learning outcomes are presented at the beginning of each session but are not discussed in detail. The course content has been adjusted and pared down from previous years based on student feedback.
Recommended texts for the course include Tipler & Mosca's ‘Physics for Scientists and Engineers’, Blundell & Blundell's ‘Concepts in Thermal Physics’, and Flowers & Mendoza's ‘Properties of Matter’. G. C. King's ‘Physics of Matter’ is particularly recommended as it aligns closely with the course content, though an eBook version is not currently available. This introductory lecture contains mostly non-examinable material, but students are expected to be proficient in basic calculations involving atomic masses, moles, and atomic sizes. Materials physics and condensed matter represent a significant research area with numerous career opportunities, including summer internships (typically for penultimate-year students), MSc programmes in Nuclear Science & Engineering, and PhDs. Information on these opportunities can be found on the third-floor noticeboard.
⚠️ Exam Alert! The lecturer explicitly stated: "Sometimes I’ll give away easter eggs, perhaps as to what might be in the exam, in order to give some advantage, at least to those that turn up to the lectures." Attending lectures may provide insights into examinable content.
1) From single-particle mechanics to many-body physics

Unlike classical mechanics, which typically deals with the deterministic behaviour of single particles or a small number of bodies, the study of many-body systems encountered in real materials and macroscopic matter requires a different approach due to the overwhelming number of degrees of freedom. Instead of tracking individual particles, new strategies involve using averaged, emergent quantities, such as temperature, to describe the collective behaviour of the system. This collective behaviour often gives rise to emergent phenomena that are not predictable from the properties of individual constituents alone. Historically, the shift from deterministic few-body physics (Newton, Laplace) to statistical and thermodynamic descriptions (Fourier, Clausius, Maxwell, Boltzmann, Kelvin) was driven by the need to understand complex systems like heat and gases.
2) Emergence in practice: intuition before equations

The concept of emergence highlights how many interacting units can produce stable, large-scale patterns that are not evident from the behaviour of individual components alone. For instance, the intricate structures formed by ants working cooperatively, such as living bridges, serve as a biological analogy for collective behaviour. This intuition can be extended to physical materials, where properties like magnetism, superconductivity, and electronic conductivity arise from the collective behaviour of electrons and atomic-scale interactions, rather than being simple sums of individual particle properties. Understanding these phenomena often begins with a physical appreciation before formal mathematical descriptions are introduced.
3) Demonstrations of thermodynamic and collective transitions
3.1 Ferromagnetism and the Curie temperature (Nickel + magnet + heat)
When a piece of nickel, an elemental ferromagnet, is heated above its Curie temperature, it detaches from a permanent magnet. As it cools, it reattaches, demonstrating a reversible thermodynamic transition. In ferromagnetic materials, microscopic magnetic moments align, causing strong attraction. However, heating injects thermal energy, randomising these moments above the Curie temperature and causing the material to transition to a paramagnetic state, where it exhibits weak or no strong attraction.
Shape-memory alloys, such as those used in a deformed paperclip, return to a pre-programmed shape when heated. This behaviour is due to a thermodynamic phase transition between two crystal structures. At low temperatures, the alloy is easily deformed without permanent bond breaking, as its structure accommodates deformation through internal "twinning." Upon heating, it transitions to a high-temperature cubic phase with a single preferred configuration, restoring the memorised shape.
3.3 Type II superconductivity and flux pinning (YBCO + magnet + liquid nitrogen)
A magnet can levitate stably over a cooled YBCO (Yttrium Barium Copper Oxide) puck, and even be "locked" in position or inverted, due to Type II superconductivity and flux pinning. Superconductors exhibit zero electrical resistance and strong diamagnetism (the Meissner effect), expelling magnetic fields. Type II superconductors, however, allow quantised magnetic flux lines to penetrate through discrete, non-superconducting regions. These flux lines become pinned within the material, stabilising the levitation and fixing the magnet's position relative to the superconductor. The discovery of "high-temperature" superconductors (above $77 \, \text{K}$) is economically significant, as it allows for cooling with cheaper liquid nitrogen instead of liquid helium, though the full mechanism behind high-temperature superconductivity remains an active area of research.
4) The atomic picture: a brief historical scaffold
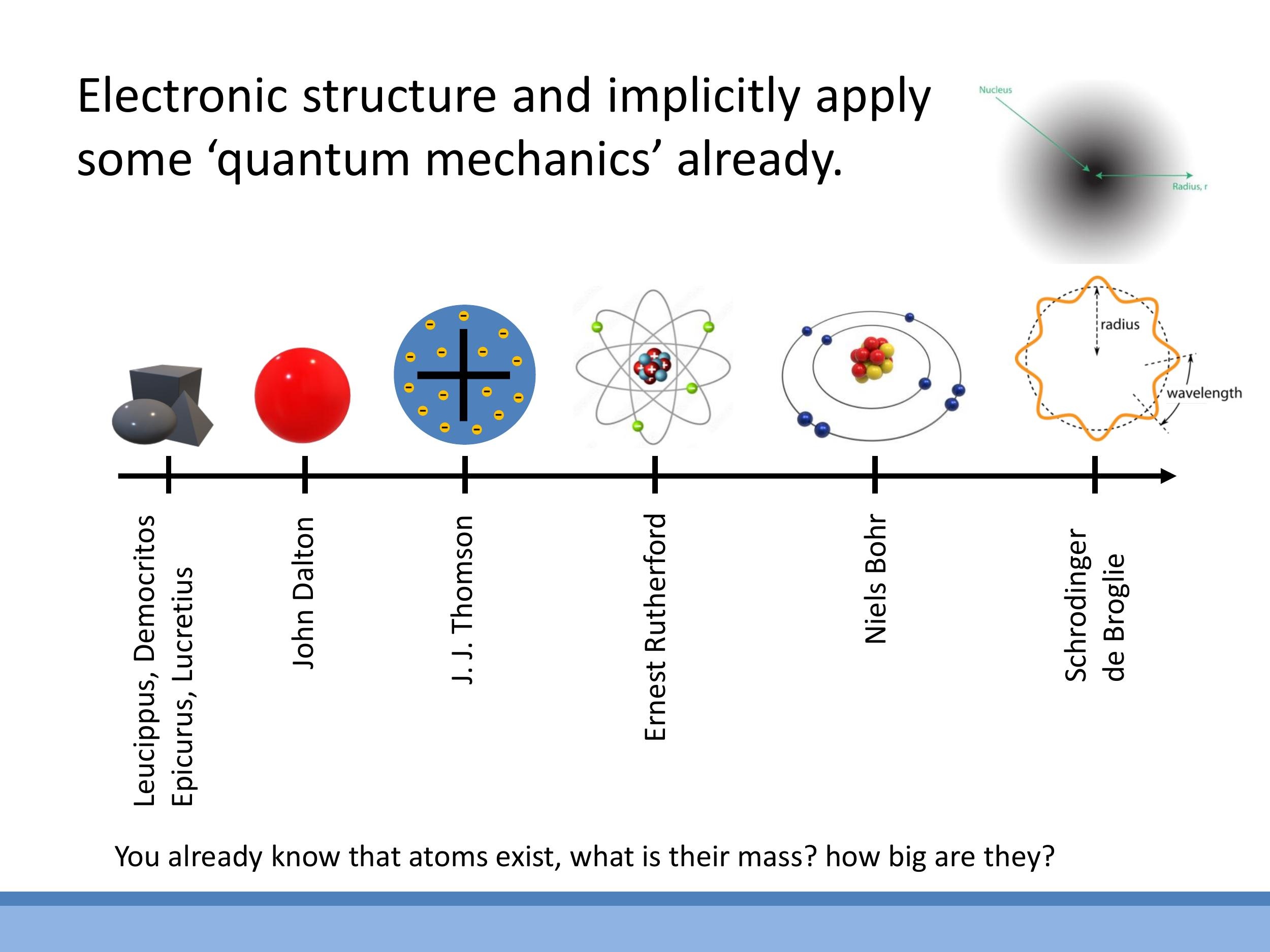
The understanding of the atom has evolved significantly over time. Philosophical atomism, dating back to ancient Greece, posited indivisible particles. Modern atomic theory began with John Dalton in the early 19th century. Subsequent models included J.J. Thomson's "plum pudding" model, Ernest Rutherford's discovery of the small, dense atomic nucleus, and Niels Bohr's model of quantised electron orbits. The contemporary view of the atom is rooted in quantum mechanics, where the nucleus (comprising protons and neutrons) is surrounded by electrons described by probabilistic orbitals with discrete energy levels, rather than classical planetary orbits.
5) Quantitative foundations: mass, moles, and atomic size
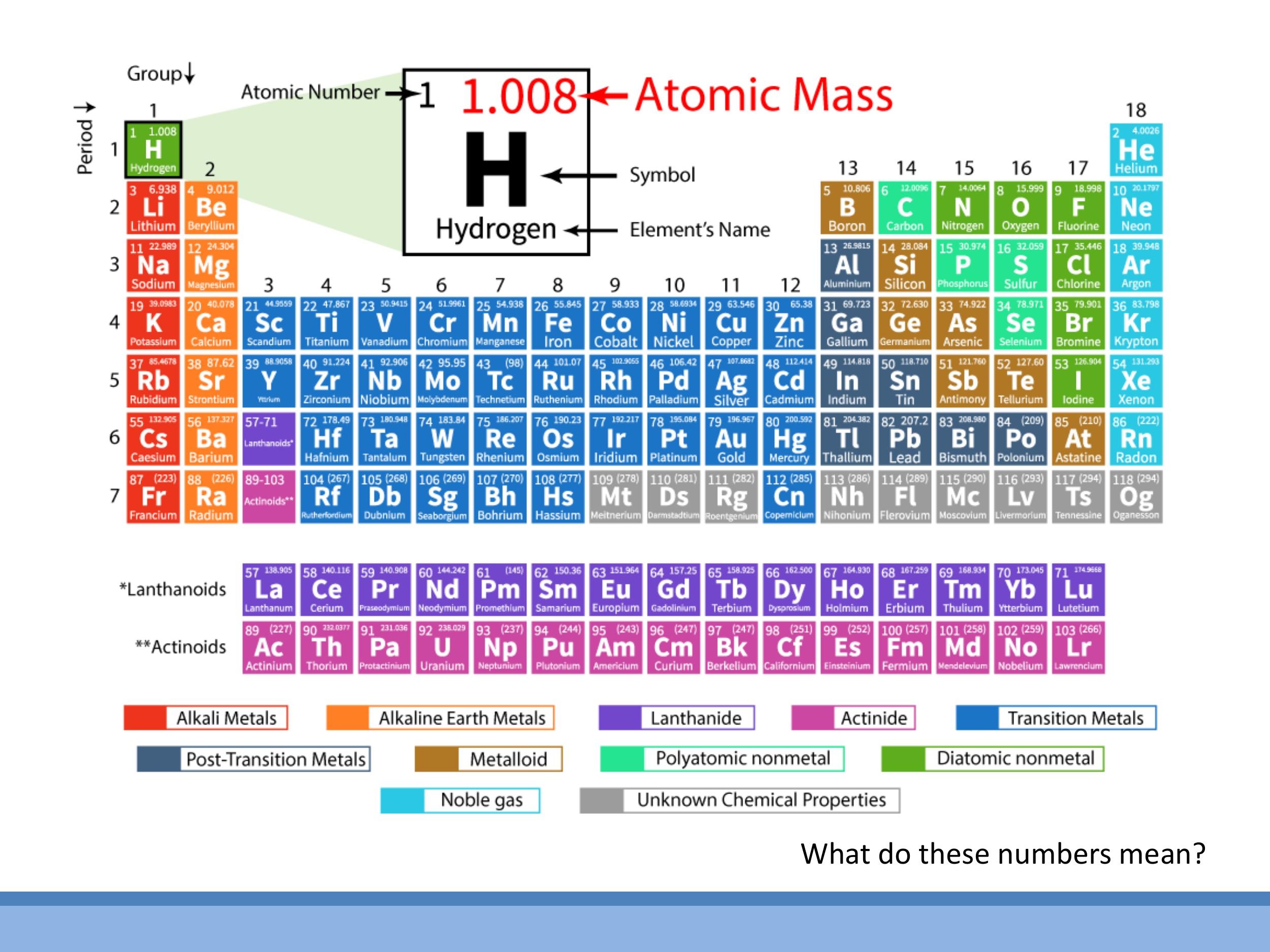
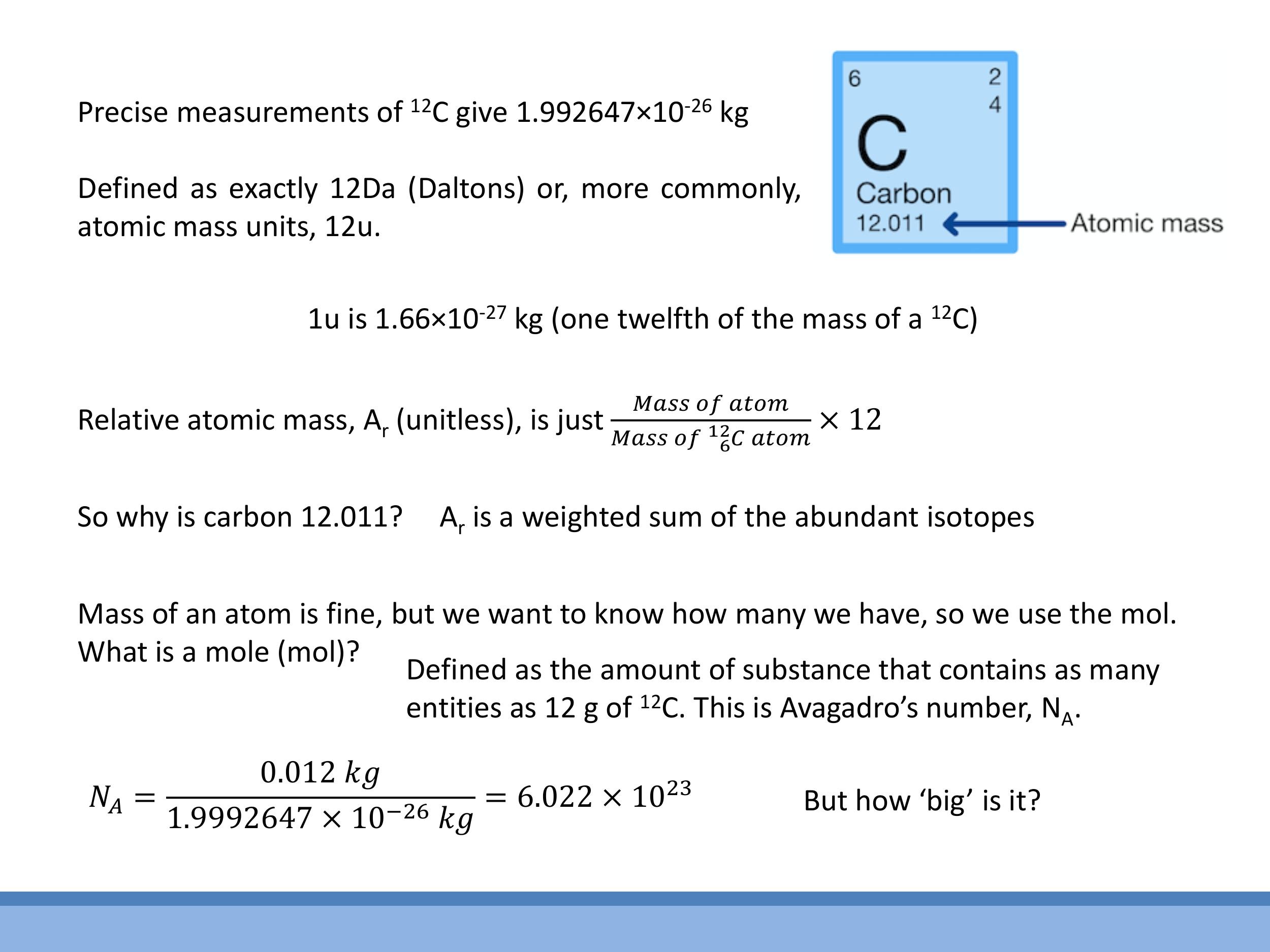
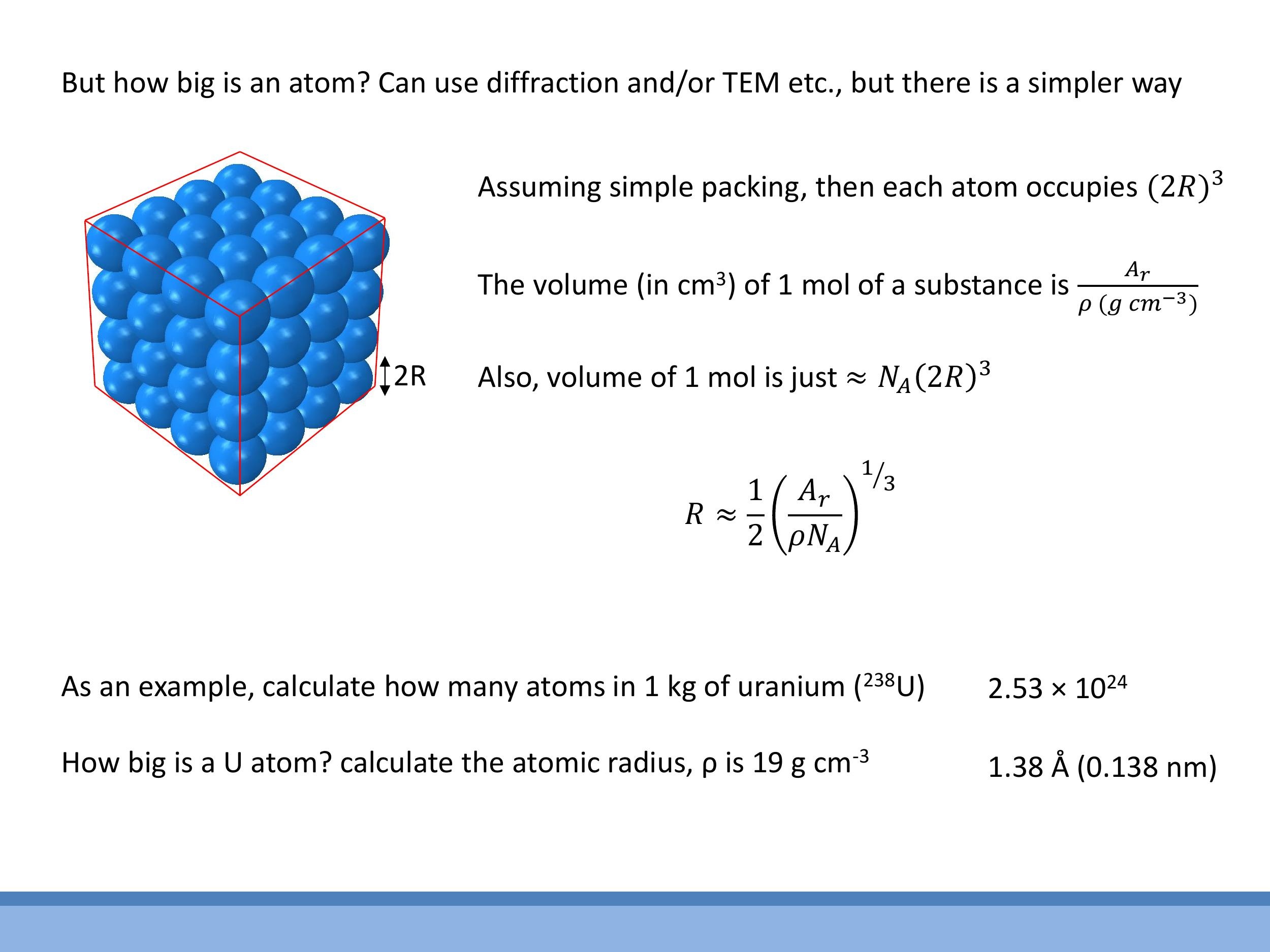
5.1 Atomic mass units and isotopes
The atomic mass unit ($\text{u}$), also known as the Dalton ($\text{Da}$), is defined such that one $^{12}\text{C}$ atom has a mass of exactly $12 \, \text{u} $, with $ 1 \, \text{u} \approx 1.66 \times 10^{-27} \, \text{kg} $. The relative atomic mass ($ A_r$) is a unitless quantity calculated as:
$$
A_r = \frac{\text{Mass of atom}}{\text{Mass of } ^{12}\text{C atom}} \times 12
$$
Atomic masses listed in the periodic table are often non-integers (e.g., carbon is $12.011$) because they represent a weighted average of the naturally occurring isotopes of an element, such as $^{12}\text{C}$ and $^{13}\text{C}$.
5.2 Counting particles: the mole and Avogadro’s number
A mole ($\text{mol}$) is defined as the amount of substance that contains as many elementary entities as there are atoms in $12 \, \text{g} $ of $ ^{12}\text{C} $. This definition leads to Avogadro's number ($ N_A $), which is approximately $ 6.022 \times 10^{23} \, \text{mol}^{-1}$. The mole and Avogadro's number provide a practical link between macroscopic quantities, such as mass in grams or volume in cubic centimetres, and the microscopic number of atoms or molecules.
5.3 Estimating how big an atom is (simple cubic packing model)
To estimate atomic size, atoms can be modelled as hard spheres of radius $R$ arranged in a simple cubic packing, where each atom occupies a cubic volume of $(2R)^3$. The molar volume ($V_m$) of a substance can be determined macroscopically from its relative atomic mass ($A_r$) and density ($\rho$) as $V_m = A_r / \rho$ (in $\text{cm}^3\text{/mol}$, with $\rho$ in $\text{g/cm}^3$). Microscopically, the molar volume can be approximated as $V_m \approx N_A (2R)^3$. Equating these two expressions allows for an estimation of the atomic radius:
$$
R \approx \frac{1}{2} \left( \frac{A_r}{\rho N_A} \right)^{1/3}
$$
For example, using uranium with $A_r = 238 \, \text{g/mol} $ and $ \rho = 19 \, \text{g/cm}^3 $, this model yields an estimated radius of $ R \approx 1.38 \, \text{Å} $ (or $ 0.138 \, \text{nm} $). This crude approximation is reasonably close to experimental values (e.g., approximately $ 1.75 \, \text{Å}$ for uranium), demonstrating the utility of simplified models in gaining physical insight.
6) The modelling mindset: “close enough” is often powerful
In the study of Properties of Matter, exact microscopic detail is frequently unnecessary for many problems. Well-chosen approximations and "back-of-the-envelope" calculations can produce useful, testable estimates that provide significant physical insight. This approach involves linking macroscopic observables to microscopic models, often with simplifying assumptions, and then validating these models by comparing their predictions with experimental data. This iterative process of estimation, measurement, and refinement is a core problem-solving strategy in physics.
Appendix: Semi-empirical Mass Formula (SEMF)
Side Note: This material is supplementary and won't be examined, but provides useful context.
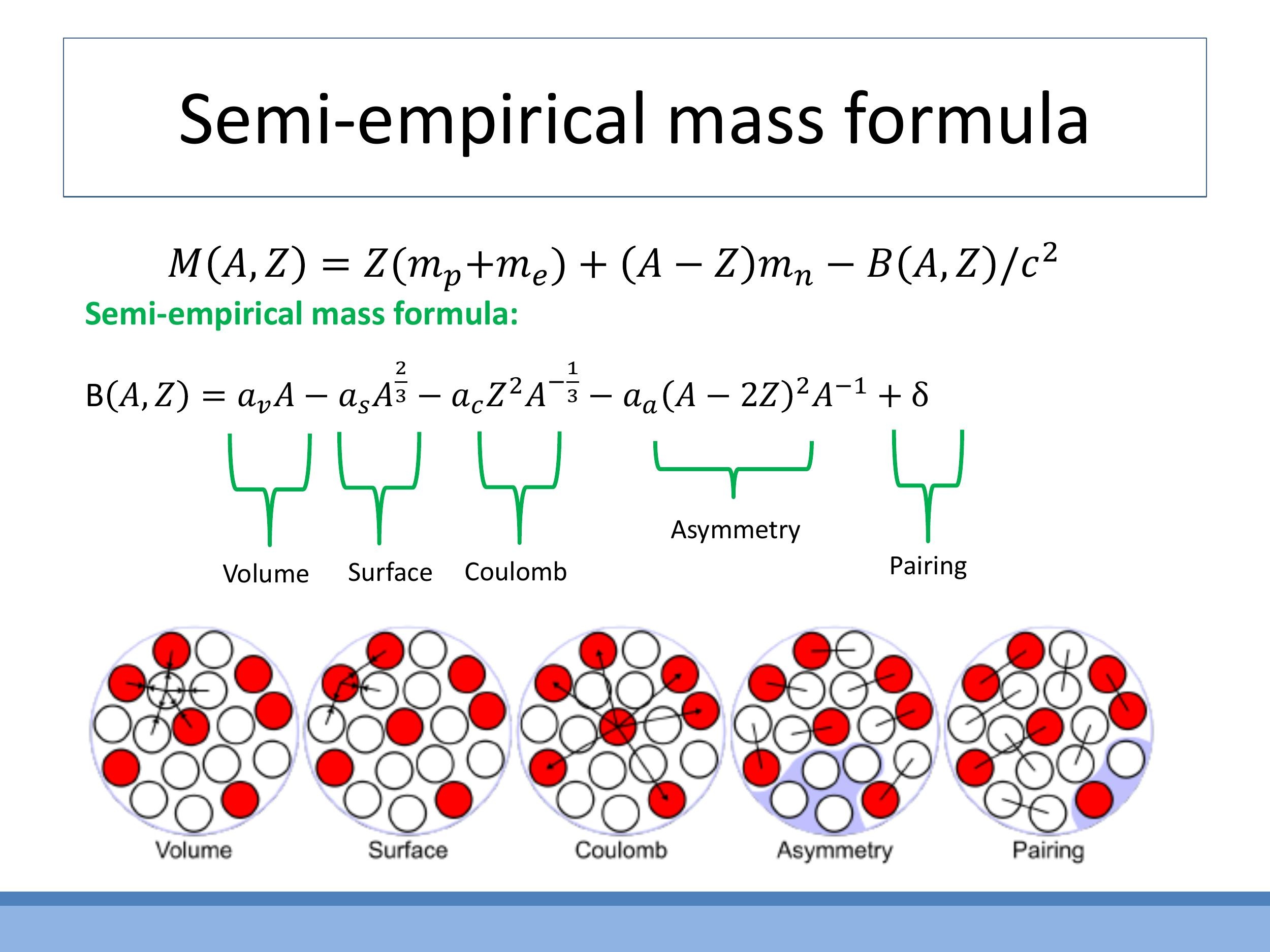
The Semi-empirical Mass Formula (SEMF) is a nuclear physics model, inspired by the liquid-drop model, used to approximate the binding energy $B(A, Z)$ of an atomic nucleus based on its mass number ($A$) and atomic number ($Z$). The formula is given by:
$$
B(A, Z) = a_v A - a_s A^{2/3} - a_c \frac{Z^2}{A^{1/3}} - a_a \frac{(A - 2Z)^2}{A} + \delta
$$
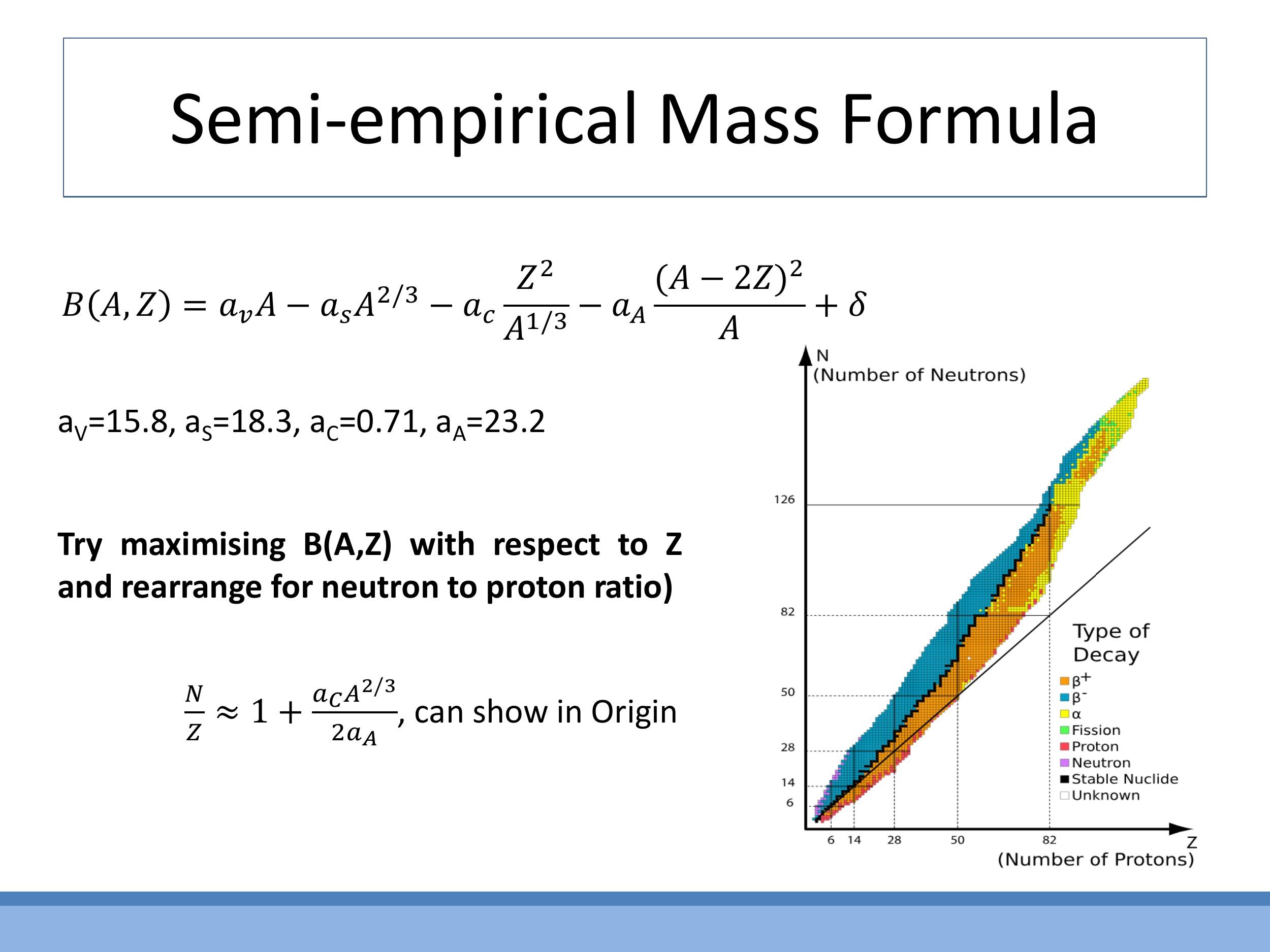
Each term in the formula has a physical interpretation: $a_v A$ represents the bulk strong-force binding (volume term); $-a_s A^{2/3}$ accounts for reduced binding at the nuclear surface (surface term); $-a_c Z^2 / A^{1/3}$ describes the electrostatic repulsion between protons (Coulomb term); $-a_a (A - 2Z)^2 / A$ penalises an imbalance between neutrons and protons (asymmetry term); and $\delta$ provides extra stability for paired nucleons (pairing term). This formula qualitatively explains the valley of stability and trends in the neutron-to-proton ratio ($N/Z$) with increasing mass number, illustrating the power of such models to describe complex phenomena.
Key takeaways
The Properties of Matter course shifts focus from few-body mechanics to many-body systems, where averaging and emergent phenomena are central. Temperature serves as a practical, everyday example of a many-body average, and collective phenomena such as magnetism, superconductivity, and shape-memory transitions are real and observable. The atomic structure consists of a nucleus and electrons; the modern electron picture is quantum mechanical and probabilistic. Essential quantitative tools for the course include understanding the atomic mass unit ($\text{u}$ or $\text{Da}$), relative atomic mass ($A_r$), the mole, and Avogadro's number ($N_A$), along with how isotopic abundances lead to non-integer $A_r$ values. Simple packing models can link bulk density and $A_r$ to an estimate of atomic radius, which, while approximate, is sufficiently accurate to be useful. The course encourages a modelling mindset, where approximations are often powerful tools for gaining physical insight quickly. Administratively, students are expected to take their own lecture notes, with slides and derivations uploaded after each session, and recommended texts are provided. Attending lectures can offer exam advantages through "easter eggs" or hints from the lecturer.
## Lecture 1: Introduction and ‘Atomos’
### 0) Orientation: How this course works and what to expect


The Properties of Matter (PoM) course, taught by Dr Ross Springell, an Associate Professor specialising in solid-state and condensed matter physics, focuses on many-particle systems such as gases, liquids, and solids, contrasting with the single-particle focus of classical mechanics. Dr Springell's research includes fundamental physics of heavy elements (actinides like uranium and thorium), nuclear fission fuels, nuclear waste materials, and tritium storage for fusion. Students are expected to maintain their own notes, as no pre-written lecture notes are provided; however, slides and handwritten derivations will be uploaded to Blackboard after each lecture. Learning outcomes are presented at the beginning of each session but are not discussed in detail. The course content has been adjusted and pared down from previous years based on student feedback.
Recommended texts for the course include Tipler & Mosca's ‘Physics for Scientists and Engineers’, Blundell & Blundell's ‘Concepts in Thermal Physics’, and Flowers & Mendoza's ‘Properties of Matter’. G. C. King's ‘Physics of Matter’ is particularly recommended as it aligns closely with the course content, though an eBook version is not currently available. This introductory lecture contains mostly non-examinable material, but students are expected to be proficient in basic calculations involving atomic masses, moles, and atomic sizes. Materials physics and condensed matter represent a significant research area with numerous career opportunities, including summer internships (typically for penultimate-year students), MSc programmes in Nuclear Science & Engineering, and PhDs. Information on these opportunities can be found on the third-floor noticeboard.
> **⚠️ Exam Alert!** The lecturer explicitly stated: "Sometimes I’ll give away easter eggs, perhaps as to what might be in the exam, in order to give some advantage, at least to those that turn up to the lectures." Attending lectures may provide insights into examinable content.
### 1) From single-particle mechanics to many-body physics

Unlike classical mechanics, which typically deals with the deterministic behaviour of single particles or a small number of bodies, the study of many-body systems encountered in real materials and macroscopic matter requires a different approach due to the overwhelming number of degrees of freedom. Instead of tracking individual particles, new strategies involve using averaged, emergent quantities, such as temperature, to describe the collective behaviour of the system. This collective behaviour often gives rise to emergent phenomena that are not predictable from the properties of individual constituents alone. Historically, the shift from deterministic few-body physics (Newton, Laplace) to statistical and thermodynamic descriptions (Fourier, Clausius, Maxwell, Boltzmann, Kelvin) was driven by the need to understand complex systems like heat and gases.
### 2) Emergence in practice: intuition before equations

The concept of emergence highlights how many interacting units can produce stable, large-scale patterns that are not evident from the behaviour of individual components alone. For instance, the intricate structures formed by ants working cooperatively, such as living bridges, serve as a biological analogy for collective behaviour. This intuition can be extended to physical materials, where properties like magnetism, superconductivity, and electronic conductivity arise from the collective behaviour of electrons and atomic-scale interactions, rather than being simple sums of individual particle properties. Understanding these phenomena often begins with a physical appreciation before formal mathematical descriptions are introduced.
### 3) Demonstrations of thermodynamic and collective transitions
#### 3.1 Ferromagnetism and the Curie temperature (Nickel + magnet + heat)
When a piece of nickel, an elemental ferromagnet, is heated above its Curie temperature, it detaches from a permanent magnet. As it cools, it reattaches, demonstrating a reversible thermodynamic transition. In ferromagnetic materials, microscopic magnetic moments align, causing strong attraction. However, heating injects thermal energy, randomising these moments above the Curie temperature and causing the material to transition to a paramagnetic state, where it exhibits weak or no strong attraction.
#### 3.2 Shape-memory alloys (deform → hot water → original shape)
Shape-memory alloys, such as those used in a deformed paperclip, return to a pre-programmed shape when heated. This behaviour is due to a thermodynamic phase transition between two crystal structures. At low temperatures, the alloy is easily deformed without permanent bond breaking, as its structure accommodates deformation through internal "twinning." Upon heating, it transitions to a high-temperature cubic phase with a single preferred configuration, restoring the memorised shape.
#### 3.3 Type II superconductivity and flux pinning (YBCO + magnet + liquid nitrogen)
A magnet can levitate stably over a cooled YBCO (Yttrium Barium Copper Oxide) puck, and even be "locked" in position or inverted, due to Type II superconductivity and flux pinning. Superconductors exhibit zero electrical resistance and strong diamagnetism (the Meissner effect), expelling magnetic fields. Type II superconductors, however, allow quantised magnetic flux lines to penetrate through discrete, non-superconducting regions. These flux lines become pinned within the material, stabilising the levitation and fixing the magnet's position relative to the superconductor. The discovery of "high-temperature" superconductors (above $77\,\text{K}$) is economically significant, as it allows for cooling with cheaper liquid nitrogen instead of liquid helium, though the full mechanism behind high-temperature superconductivity remains an active area of research.
### 4) The atomic picture: a brief historical scaffold

The understanding of the atom has evolved significantly over time. Philosophical atomism, dating back to ancient Greece, posited indivisible particles. Modern atomic theory began with John Dalton in the early 19th century. Subsequent models included J.J. Thomson's "plum pudding" model, Ernest Rutherford's discovery of the small, dense atomic nucleus, and Niels Bohr's model of quantised electron orbits. The contemporary view of the atom is rooted in quantum mechanics, where the nucleus (comprising protons and neutrons) is surrounded by electrons described by probabilistic orbitals with discrete energy levels, rather than classical planetary orbits.
### 5) Quantitative foundations: mass, moles, and atomic size



#### 5.1 Atomic mass units and isotopes
The atomic mass unit ($\text{u}$), also known as the Dalton ($\text{Da}$), is defined such that one $^{12}\text{C}$ atom has a mass of exactly $12\,\text{u}$, with $1\,\text{u} \approx 1.66 \times 10^{-27}\,\text{kg}$. The relative atomic mass ($A_r$) is a unitless quantity calculated as:
$$ A_r = \frac{\text{Mass of atom}}{\text{Mass of } ^{12}\text{C atom}} \times 12 $$
Atomic masses listed in the periodic table are often non-integers (e.g., carbon is $12.011$) because they represent a weighted average of the naturally occurring isotopes of an element, such as $^{12}\text{C}$ and $^{13}\text{C}$.
#### 5.2 Counting particles: the mole and Avogadro’s number
A mole ($\text{mol}$) is defined as the amount of substance that contains as many elementary entities as there are atoms in $12\,\text{g}$ of $^{12}\text{C}$. This definition leads to Avogadro's number ($N_A$), which is approximately $6.022 \times 10^{23}\,\text{mol}^{-1}$. The mole and Avogadro's number provide a practical link between macroscopic quantities, such as mass in grams or volume in cubic centimetres, and the microscopic number of atoms or molecules.
#### 5.3 Estimating how big an atom is (simple cubic packing model)
To estimate atomic size, atoms can be modelled as hard spheres of radius $R$ arranged in a simple cubic packing, where each atom occupies a cubic volume of $(2R)^3$. The molar volume ($V_m$) of a substance can be determined macroscopically from its relative atomic mass ($A_r$) and density ($\rho$) as $V_m = A_r / \rho$ (in $\text{cm}^3\text{/mol}$, with $\rho$ in $\text{g/cm}^3$). Microscopically, the molar volume can be approximated as $V_m \approx N_A (2R)^3$. Equating these two expressions allows for an estimation of the atomic radius:
$$ R \approx \frac{1}{2} \left( \frac{A_r}{\rho N_A} \right)^{1/3} $$
For example, using uranium with $A_r = 238\,\text{g/mol}$ and $\rho = 19\,\text{g/cm}^3$, this model yields an estimated radius of $R \approx 1.38\,\text{Å}$ (or $0.138\,\text{nm}$). This crude approximation is reasonably close to experimental values (e.g., approximately $1.75\,\text{Å}$ for uranium), demonstrating the utility of simplified models in gaining physical insight.
### 6) The modelling mindset: “close enough” is often powerful

In the study of Properties of Matter, exact microscopic detail is frequently unnecessary for many problems. Well-chosen approximations and "back-of-the-envelope" calculations can produce useful, testable estimates that provide significant physical insight. This approach involves linking macroscopic observables to microscopic models, often with simplifying assumptions, and then validating these models by comparing their predictions with experimental data. This iterative process of estimation, measurement, and refinement is a core problem-solving strategy in physics.
## Appendix: Semi-empirical Mass Formula (SEMF)


*Side Note:* This material is supplementary and won't be examined, but provides useful context.
The Semi-empirical Mass Formula (SEMF) is a nuclear physics model, inspired by the liquid-drop model, used to approximate the binding energy $B(A, Z)$ of an atomic nucleus based on its mass number ($A$) and atomic number ($Z$). The formula is given by:
$$ B(A, Z) = a_v A - a_s A^{2/3} - a_c \frac{Z^2}{A^{1/3}} - a_a \frac{(A - 2Z)^2}{A} + \delta $$
Each term in the formula has a physical interpretation: $a_v A$ represents the bulk strong-force binding (volume term); $-a_s A^{2/3}$ accounts for reduced binding at the nuclear surface (surface term); $-a_c Z^2 / A^{1/3}$ describes the electrostatic repulsion between protons (Coulomb term); $-a_a (A - 2Z)^2 / A$ penalises an imbalance between neutrons and protons (asymmetry term); and $\delta$ provides extra stability for paired nucleons (pairing term). This formula qualitatively explains the valley of stability and trends in the neutron-to-proton ratio ($N/Z$) with increasing mass number, illustrating the power of such models to describe complex phenomena.
## Key takeaways
The Properties of Matter course shifts focus from few-body mechanics to many-body systems, where averaging and emergent phenomena are central. Temperature serves as a practical, everyday example of a many-body average, and collective phenomena such as magnetism, superconductivity, and shape-memory transitions are real and observable. The atomic structure consists of a nucleus and electrons; the modern electron picture is quantum mechanical and probabilistic. Essential quantitative tools for the course include understanding the atomic mass unit ($\text{u}$ or $\text{Da}$), relative atomic mass ($A_r$), the mole, and Avogadro's number ($N_A$), along with how isotopic abundances lead to non-integer $A_r$ values. Simple packing models can link bulk density and $A_r$ to an estimate of atomic radius, which, while approximate, is sufficiently accurate to be useful. The course encourages a modelling mindset, where approximations are often powerful tools for gaining physical insight quickly. Administratively, students are expected to take their own lecture notes, with slides and derivations uploaded after each session, and recommended texts are provided. Attending lectures can offer exam advantages through "easter eggs" or hints from the lecturer.










