Lecture 2: Interatomic Forces (part 1)
0) Orientation and learning outcomes
The "Properties of Matter" (PoM) course transitions from single-particle mechanics to the behaviour of many-body systems, exploring why solids, liquids, and gases exist and how their properties arise from atomic interactions. This lecture sets the foundational atomic picture, which will later be connected to classical thermodynamics.
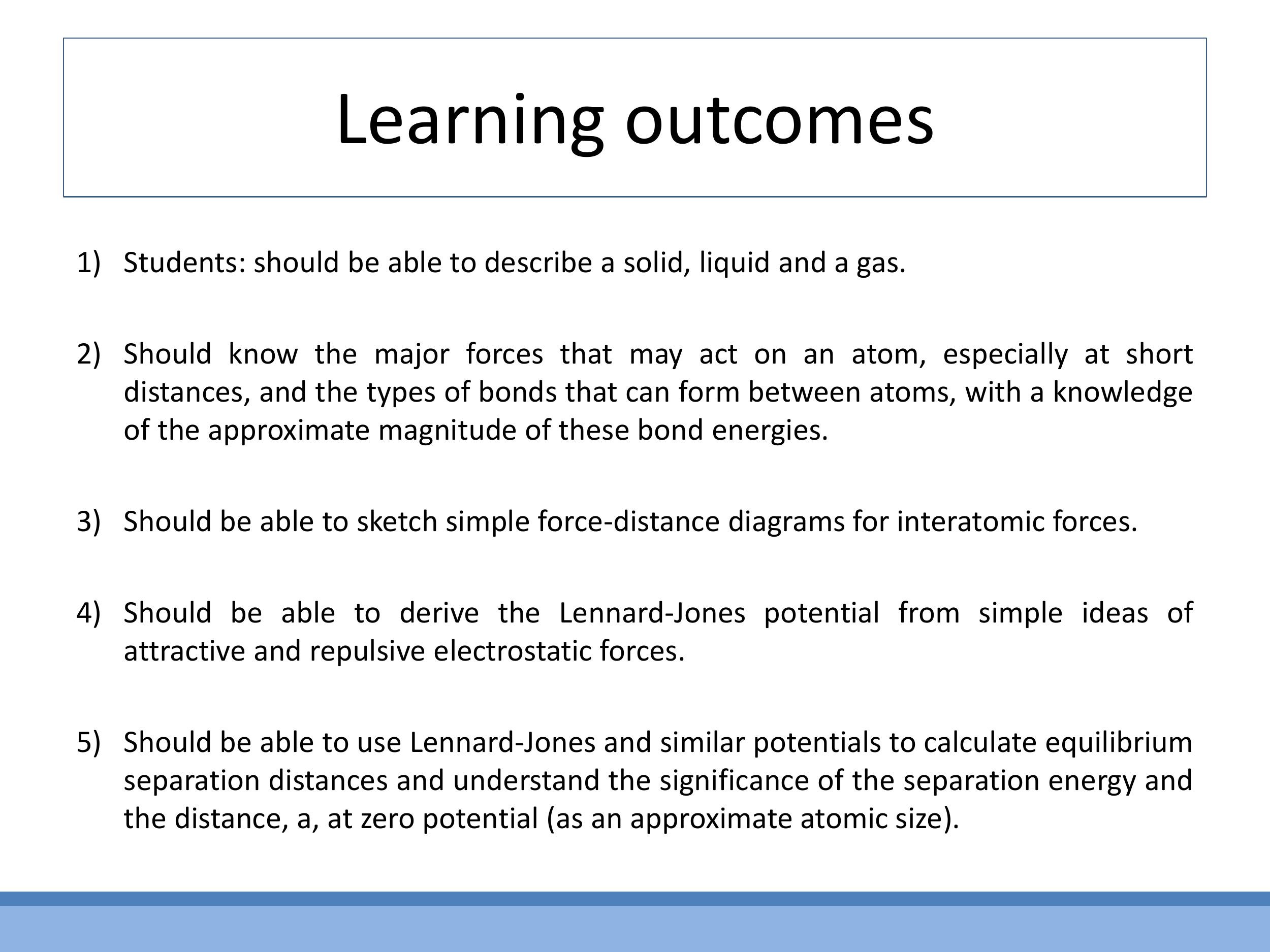
Students are expected to describe solids, liquids, and gases using physical properties such as compressibility, rigidity, and viscosity. They should understand the major interatomic forces, recognise different bond types and their typical energies, and be able to sketch qualitative force-distance curves to identify equilibrium separation. Furthermore, students will learn to derive the Lennard-Jones potential from a two-term force model and use similar potentials to determine equilibrium distances and interpret key parameters like $\varepsilon$ (well depth) and $a$ (distance at $V=0$).
Side Note: Any material marked "Appendix" throughout this course is supplementary, intended for interest only, and will not be assessed in examinations.
1) Why both attraction and repulsion must exist
The existence of condensed matter, such as solids and liquids, in everyday life unequivocally demonstrates the presence of attractive forces between atoms. Without such attraction, matter would not coalesce into stable structures. Conversely, if only attractive forces existed, all matter would collapse into an infinitely dense state. The stable, finite separations observed between atoms imply a necessary counteracting short-range repulsive force.
The dominant mechanism for this strong, short-range repulsion is the Pauli exclusion principle. This quantum mechanical rule dictates that no two electrons can occupy the same quantum state, meaning their wavefunctions cannot significantly overlap. As atoms approach each other, the overlap of electron clouds leads to a steep increase in energy, manifesting as a powerful repulsive force. While Coulombic repulsion between positively charged nuclei also exists, the Pauli exclusion principle provides the primary short-range repulsive barrier at typical interatomic distances.
This dynamic can be intuitively understood through a demonstration involving "inverter magnets." A central magnet experiences a weak, long-range attraction to surrounding magnets, creating a stable equilibrium point. However, if forced too close, a strong short-range repulsion rapidly pushes it away. If displaced from its equilibrium, the magnet "snaps back" to a stable separation, illustrating the balance between attractive and repulsive forces that establishes a stable "sweet spot" for interatomic distances.
2) States of matter: beyond the GCSE table
Traditional definitions of states of matter often rely on simplified rules that have significant exceptions. For instance, while solids are generally denser than liquids, and liquids denser than gases, water is a notable exception where ice (solid) is less dense than liquid water. Similarly, elements like silicon and germanium also exhibit this inverse density relationship. The notion that solids possess a "regular pattern" is also not universally true, as amorphous solids like glass, as well as polymers, lack long-range crystalline order.
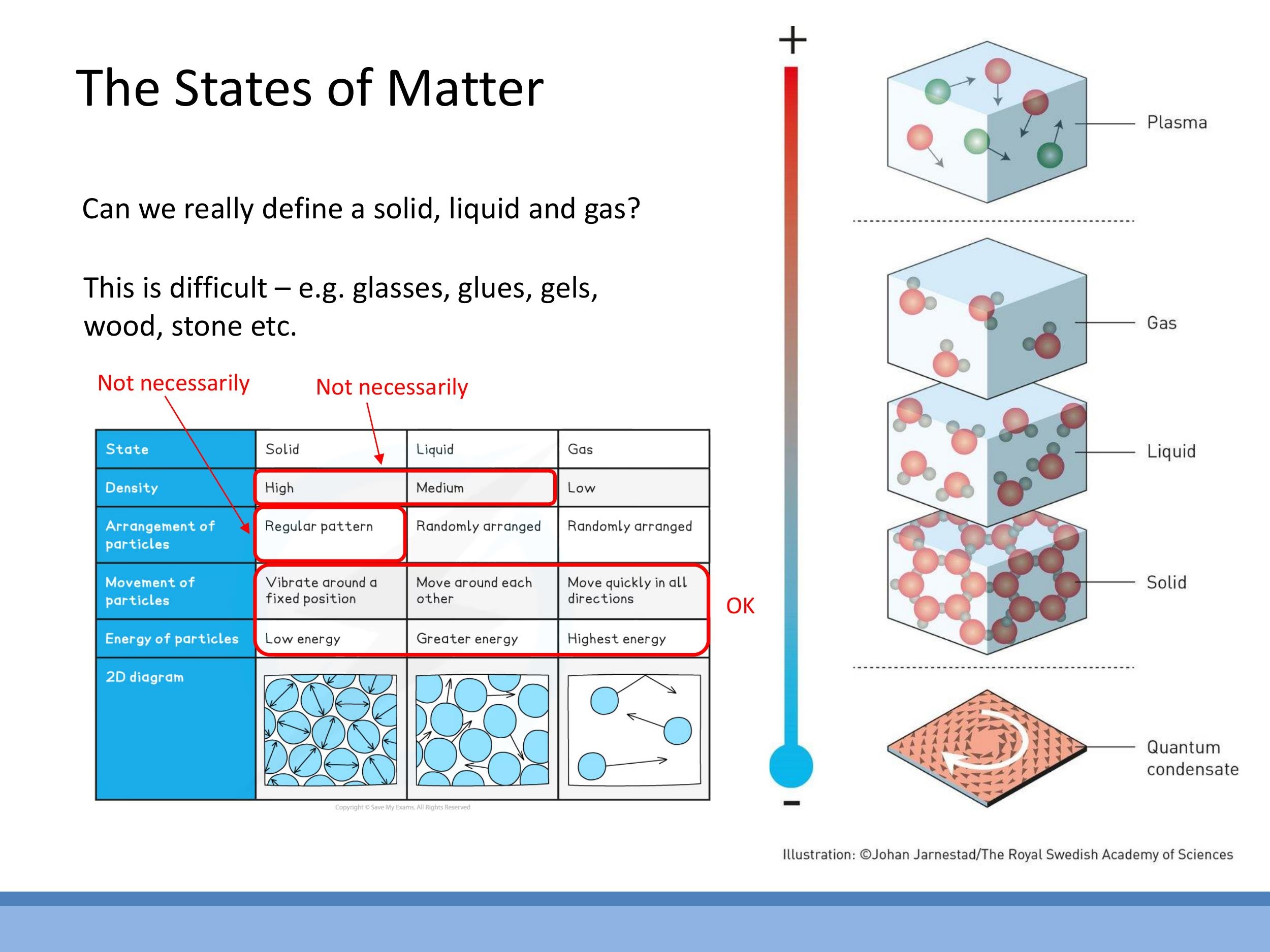
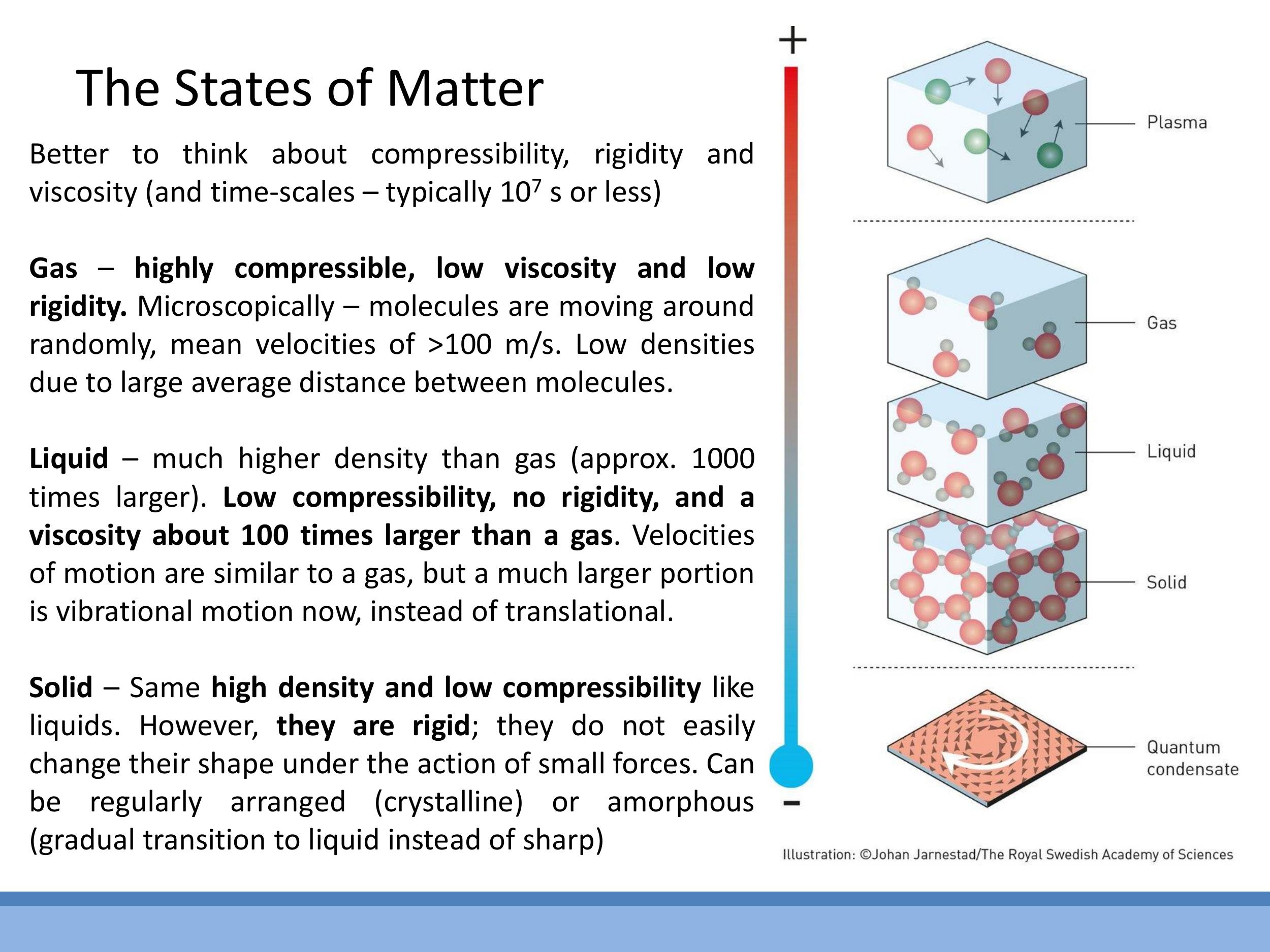
A more physically robust characterisation of matter states focuses on properties such as compressibility, rigidity, and viscosity, alongside the microscopic motion of particles.
- Gas: Highly compressible, with low rigidity and viscosity. Particles are widely separated and exhibit fast, random translational motion. For example, nitrogen molecules in air at $20 \, ^\circ\text{C} $ have a root mean square speed of approximately $ 500 \, \text{m s}^{-1}$.
- Liquid: Characterised by low compressibility and an absence of rigidity, but with viscosity typically about 100 times greater than that of a gas. Molecules are closely packed but can still move past one another, exhibiting a mixture of translational and vibrational motion.
- Solid: Rigid and possesses low compressibility, similar to liquids. Atoms in a solid primarily vibrate about fixed positions, with negligible translational motion.
While this course focuses on solid, liquid, and gas phases, it is worth noting that the vast majority of ordinary matter in the universe exists as plasma, a state where atoms are ionised.
3) Which fundamental force binds atoms? Range and scale
The four fundamental forces of nature are the strong nuclear force ($\sim 10^{-15} \, \text{m} $ range), the weak nuclear force ($ \sim 10^{-18} \, \text{m} $ range), the electromagnetic force (infinite range), and gravity (infinite range). Given that typical interatomic separations are approximately $ 10^{-10} \, \text{m} $ (one Ångström, $ 1 \, \text{Å}$), the strong and weak nuclear forces are too short-ranged to be relevant for atomic binding.
⚠️ Exam Alert! The lecturer explicitly stated: "It's useful this number because it's going to help you whenever you face questions on the multiple choice exam in December, which will definitely ask you about this kind of thing or longer questions in the summer exam... if you've got an idea already of what a sensible distance is, you'll know whether you're on the right track or not." Students should remember that atomic distances are typically of the order of $1 \, \text{Å} $ or $ 10^{-10} \, \text{m}$.
To determine the relative importance of gravity versus electromagnetism for interatomic bonding, a quantitative comparison of their potential energies can be performed. Consider two hypothetical atoms, each with a mass of $100 \, \text{u} $ (atomic mass units) and charges of $ \pm e $ (elementary charge), separated by a distance $ r = 1.5 \, \text{Å} = 1.5 \times 10^{-10} \, \text{m}$.
The gravitational potential energy $U_g$ is given by:
$$
U_g = -\frac{G m_1 m_2}{r}
$$
The electrostatic potential energy $U_e$ is given by:
$$
U_e = \frac{q_1 q_2}{4\pi\varepsilon_0 r}
$$
Calculating these values and converting to electron volts (eV) reveals $U_g \approx 7.65 \times 10^{-32} \, \text{eV} $ and $ U_e \approx 9.6 \, \text{eV} $. This demonstrates that the electromagnetic force is approximately $ 10^{32}$ times stronger than gravity at these scales, rendering gravity entirely negligible for interatomic bonding. Therefore, the electromagnetic force is the sole fundamental force responsible for holding atoms together.
4) Bond types as manifestations of electrostatics
All interatomic bonding originates from electrostatic interactions. These can be categorised into strong and weak bonds based on their energy scale.
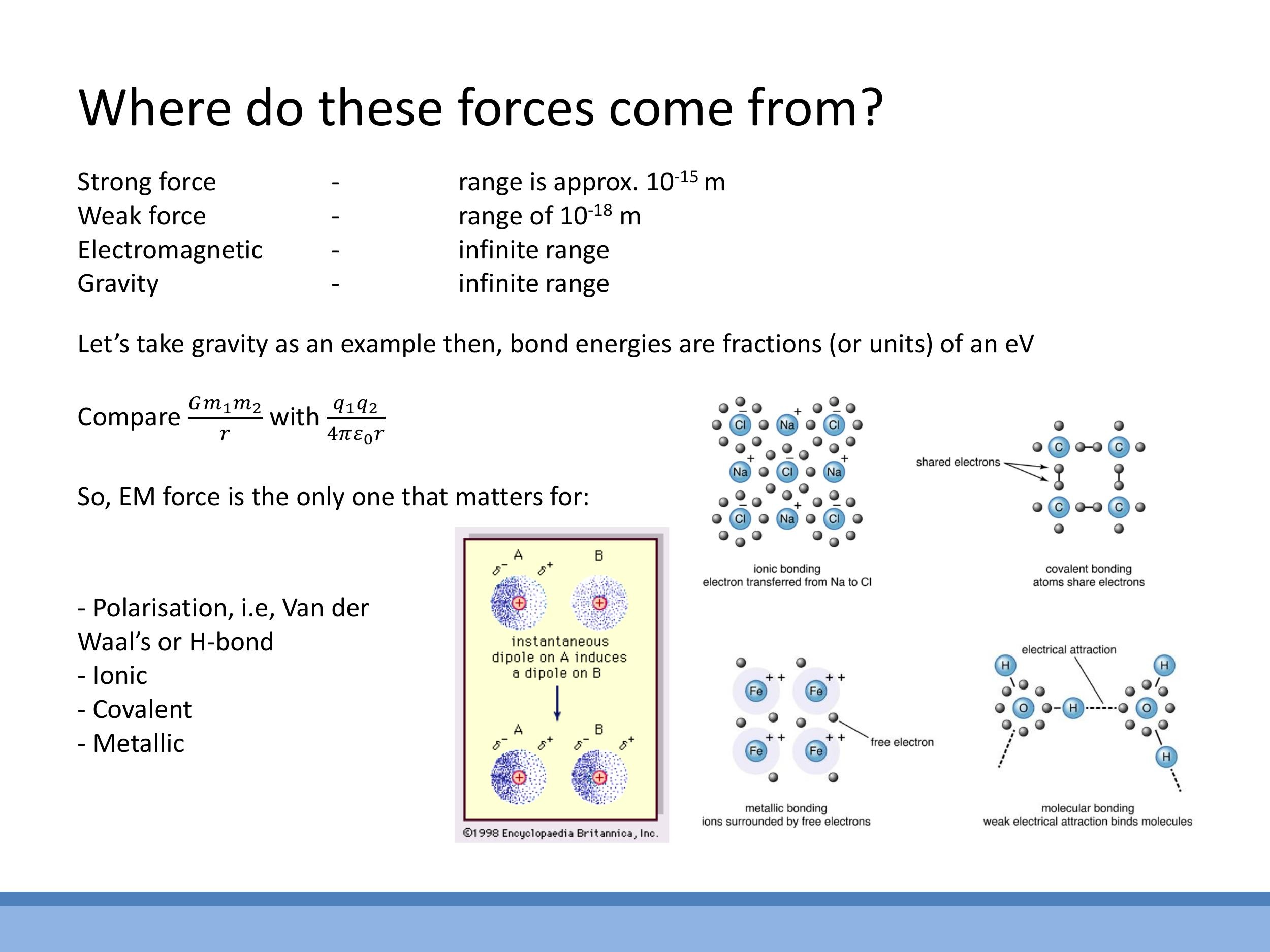
Strong bonds, typically measured in electron volts, include:
- Ionic bonds: Formed by the complete transfer of electrons from one atom to another, creating positively and negatively charged ions (cations and anions) that are held together by strong Coulombic attraction, as seen in compounds like sodium chloride ($\text{NaCl}$).
- Covalent bonds: Involve the sharing of electron pairs between atoms, leading to strong, directional bonds.
- Metallic bonds: Consist of a lattice of positive ion cores immersed in a "sea" of delocalised valence electrons, resulting in strong, non-directional bonding.
Weaker, polar interactions include:
- Hydrogen bonds: Occur between molecules containing highly electronegative atoms (like oxygen or nitrogen) bonded to hydrogen, creating permanent dipoles that attract each other.
- Van der Waals forces (London dispersion forces): Arise from instantaneous fluctuations in electron clouds, creating temporary dipoles that induce dipoles in neighbouring atoms, leading to weak, transient attractions.
5) Van der Waals forces and noble gases
Noble gases, such as helium, neon, and argon, present a puzzle because they are chemically inert with fully occupied electron shells, yet they can be liquefied by cooling. This phenomenon cannot be explained by strong ionic, covalent, or metallic bonding.
The mechanism responsible is the London dispersion force, a type of van der Waals interaction. Due to the constant motion of electrons, an instantaneous, temporary fluctuation in the electron cloud of one atom can create a transient electric dipole. This temporary dipole then induces a corresponding dipole in a nearby neutral atom, resulting in a weak, short-lived attractive force between the two atoms. This transient nature is key to their weakness.
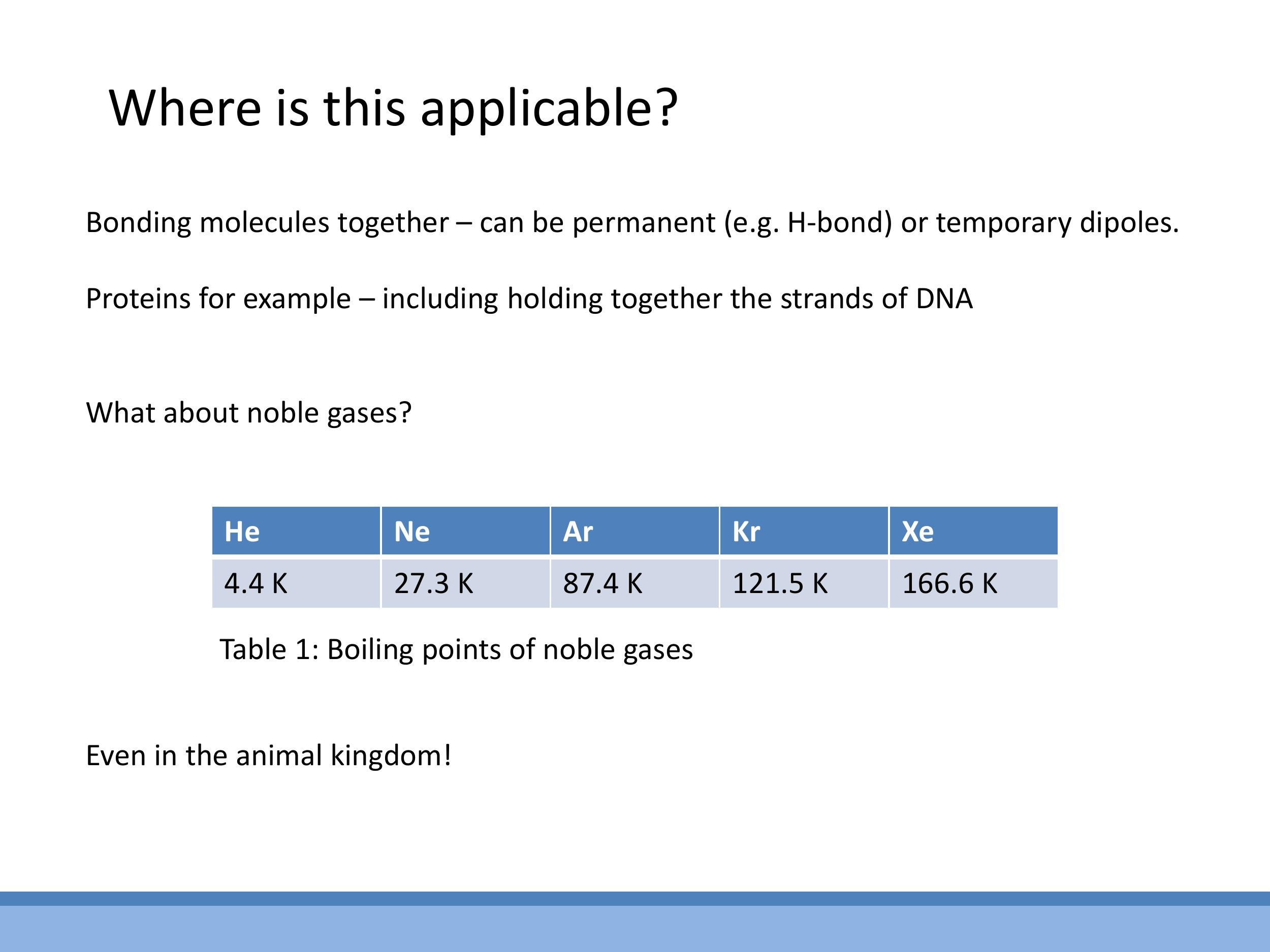
Empirical evidence supports this mechanism: the boiling points of noble gases increase progressively down the group (from helium to xenon). This trend is consistent with the increasing number of electrons and greater polarisability of larger atoms, which allows for stronger instantaneous and induced dipoles, leading to more significant van der Waals attractions and thus higher temperatures required for liquefaction.
6) A simple interatomic force model: two-term power law
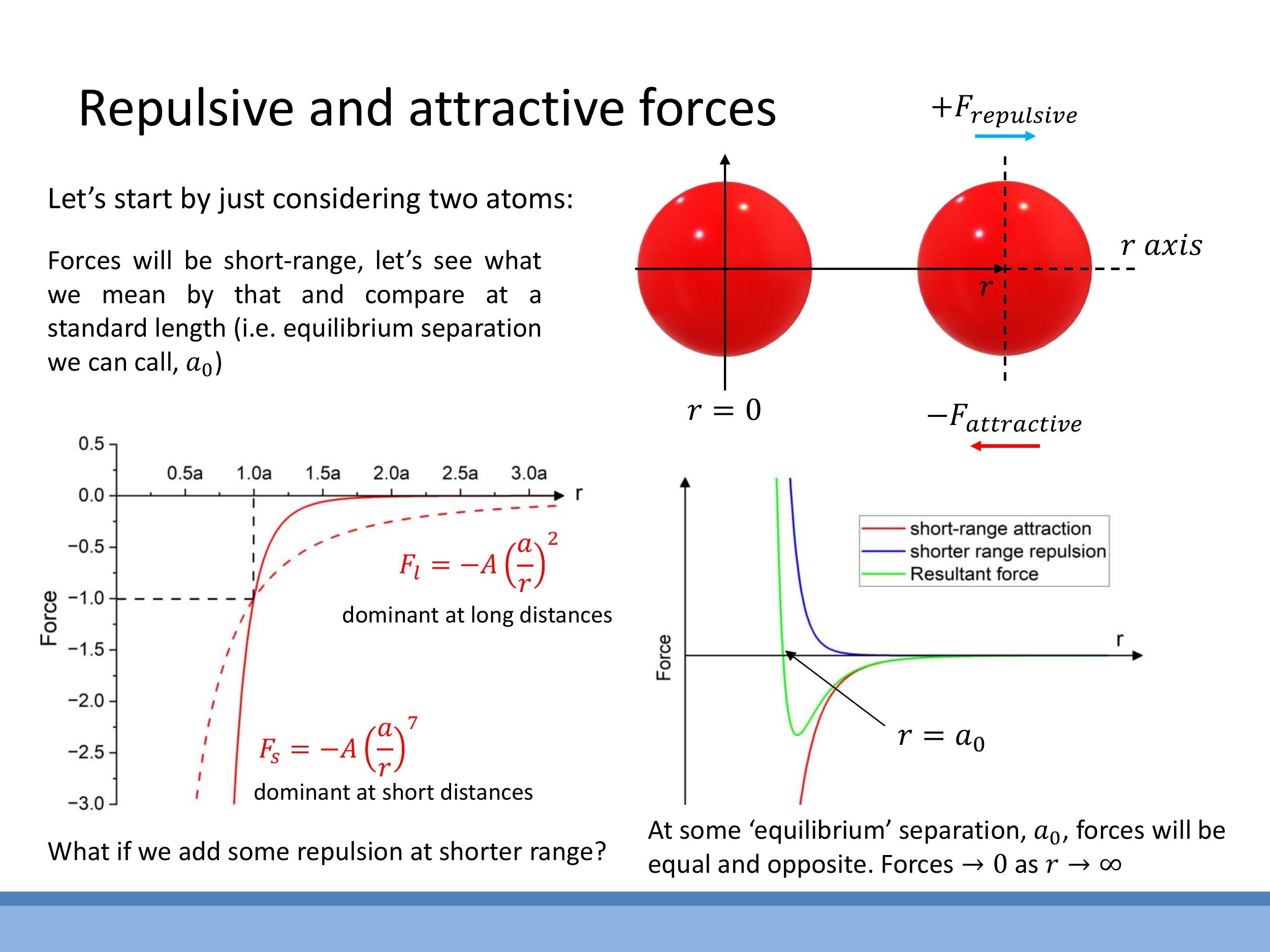
A simple yet effective model for the net interatomic force $F(r)$ between two atoms at separation $r$ combines a short-range repulsive term and a longer-range attractive term. This is often expressed as:
$$
F(r) = \frac{A}{r^m} - \frac{B}{r^n}
$$
Here, the first term, $A/r^m$, represents the repulsive force, while the second term, $-B/r^n$, represents the attractive force. The condition $m > n$ is crucial, as it ensures the repulsive force is much steeper and dominates at very short distances, preventing atomic collapse, while the attractive force has a longer range. Both forces tend to zero as $r \to \infty$.
The equilibrium separation distance, $a_0$, is defined as the point where the net force is zero, i.e., $F(a_0) = 0$. By setting $F(a_0) = 0$, a relationship between the coefficients $A$ and $B$ can be derived: $B = A a_0^{n-m}$. Substituting this back into the force equation allows $F(r)$ to be expressed in terms of $a_0$, which represents the stable "sweet spot" spacing in condensed phases. For van der Waals systems, typical exponents are $m \approx 13$ for the repulsive term and $n \approx 7$ for the attractive term. This model captures the essential qualitative features of interatomic interactions: a very steep repulsive wall at short distances (due to Pauli exclusion) and a gentler, longer-ranged attraction (e.g., van der Waals forces).
7) Near equilibrium: atoms behave like masses on springs (SHM)
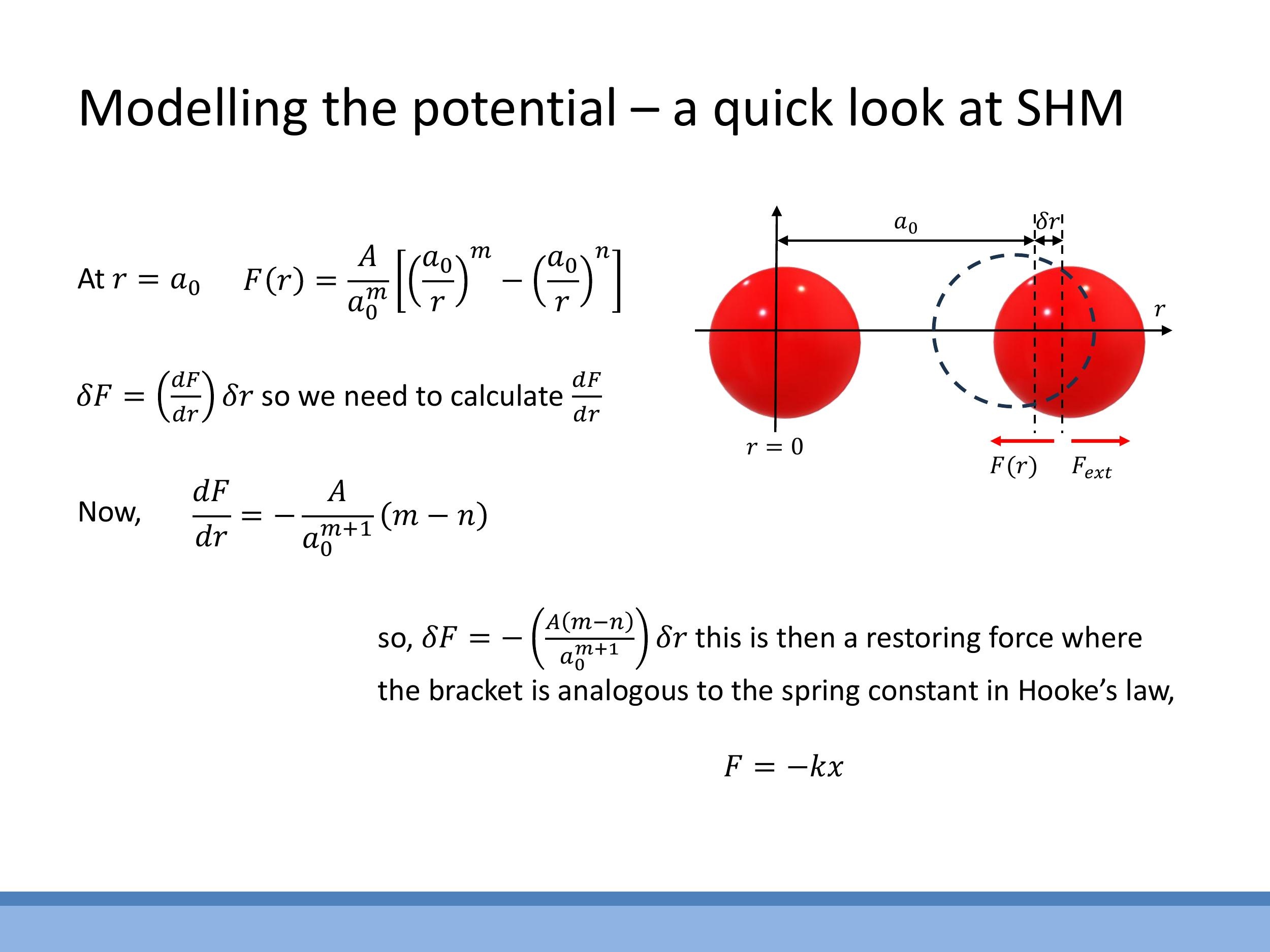
For small displacements, $\delta r$, from the equilibrium separation $a_0$, the interatomic force curve can be approximated as linear. This linearisation is analogous to Hooke's Law for a spring. The change in force, $\delta F$, for a small displacement $\delta r$ is given by the gradient of the force curve at equilibrium:
$$ \delta F = \left.\frac{dF}{dr}\right| {r=a_0} \cdot \delta r $$
This expression can be directly compared to Hooke's Law, $F = -k x$, where the effective spring constant $k \text{eff} $ is defined as the negative of the gradient of the force curve at equilibrium: $ k_\text{eff} = -\left.\frac{dF}{dr}\right|_{r=a_0} $. This implies that for small perturbations, atoms behave as if connected by springs, exerting a restoring force that brings them back to $ a_0$.
This "atoms-as-springs" model is fundamental to understanding phenomena like thermal conductivity in non-metals. In such materials, heat is primarily transported by lattice vibrations, known as phonons, which are quantised wave packets of vibrational energy propagating through the interconnected atomic network.
8) From force to potential: arriving at Lennard-Jones
The interatomic potential energy $V(r)$ is related to the force $F(r)$ by the negative integral of the force with respect to distance, with the convention that the potential energy is zero at infinite separation, $V(\infty) = 0$:
$$
V(r) = -\int F(r)\,dr
$$
Applying this integration to the two-term force model with van der Waals-like exponents ($m=13$ and $n=7$) yields the Lennard-Jones 12-6 potential:
$$
V(r) = 4\varepsilon \left[ \left(\frac{a}{r}\right)^{12} - \left(\frac{a}{r}\right)^6 \right]
$$
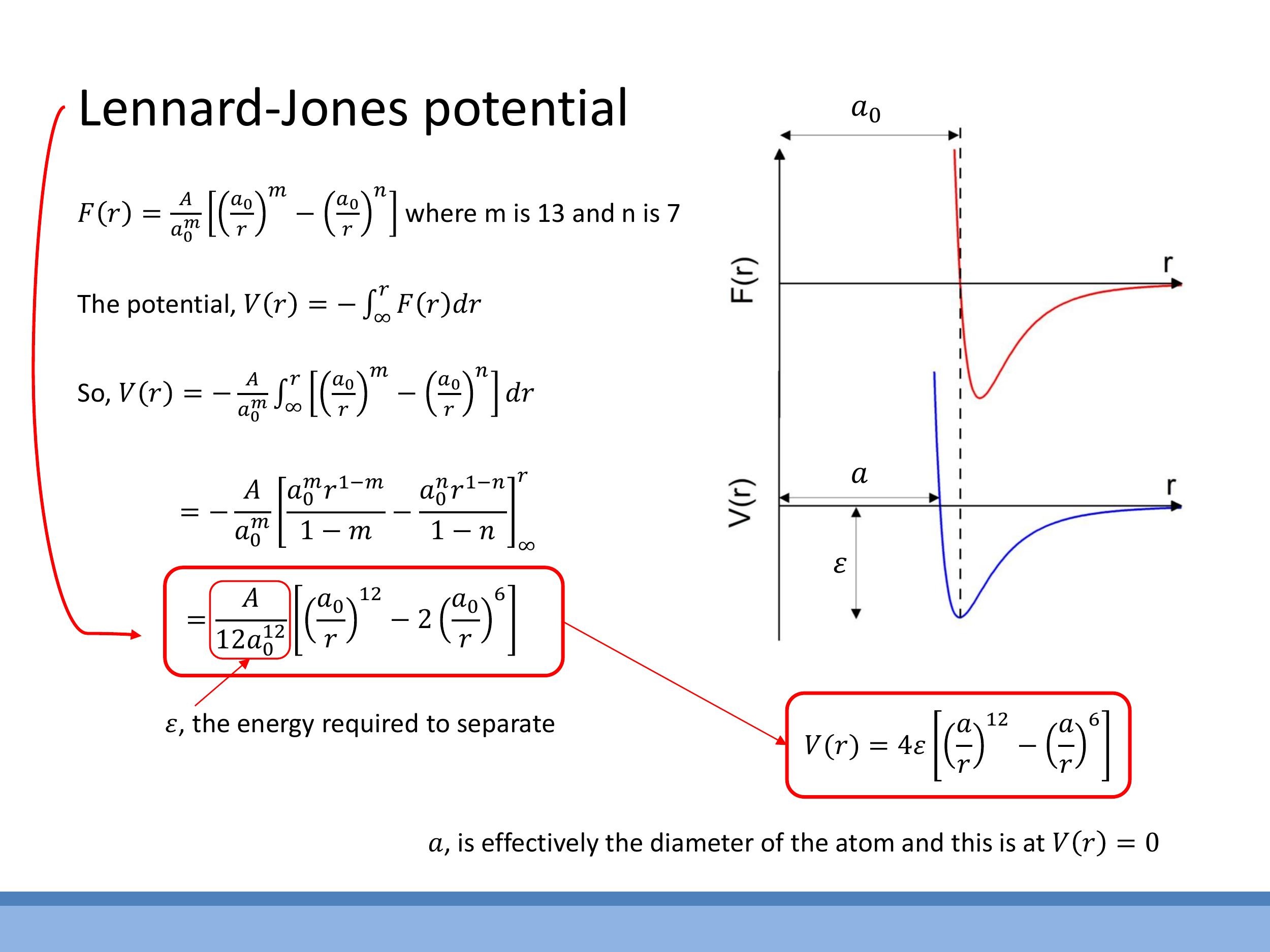
In this widely used form, $\varepsilon$ represents the depth of the potential well, which corresponds to the energy required to separate the two atoms from their bound state (i.e., the bond strength). The parameter $a$ is the distance at which the potential energy $V(r)$ is zero, often used as an approximate atomic size or scale parameter in models. The minimum of this potential, corresponding to the equilibrium separation $r_\text{eq}$ (or $a_0$ in the force model), occurs at $r_\text{eq} = 2^{1/6}a$, which is slightly greater than $a$. The potential curve graphically illustrates a steep repulsive wall at short distances, a minimum at $r_\text{eq}$, and a gradual approach to zero from below as $r$ increases.
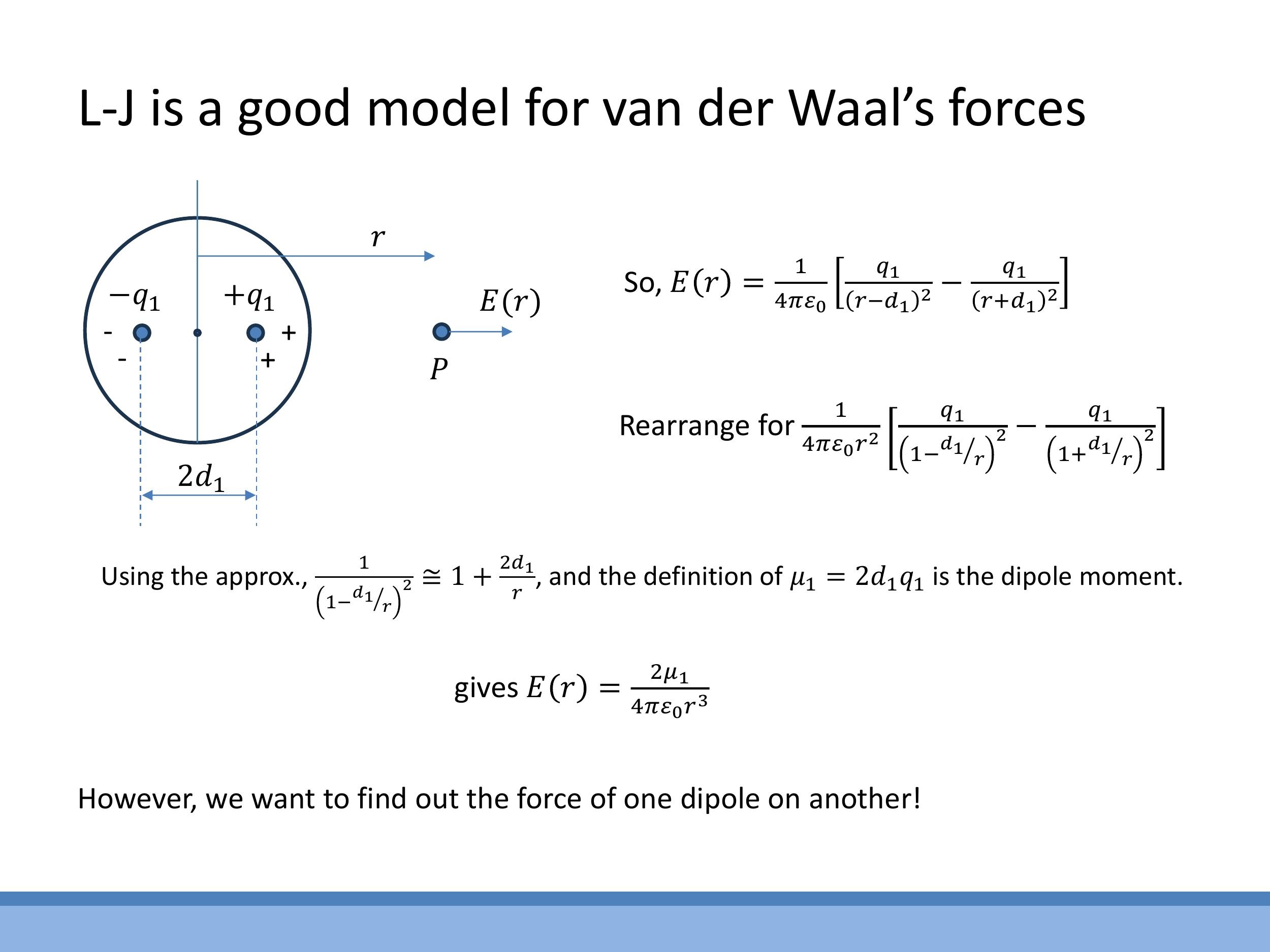
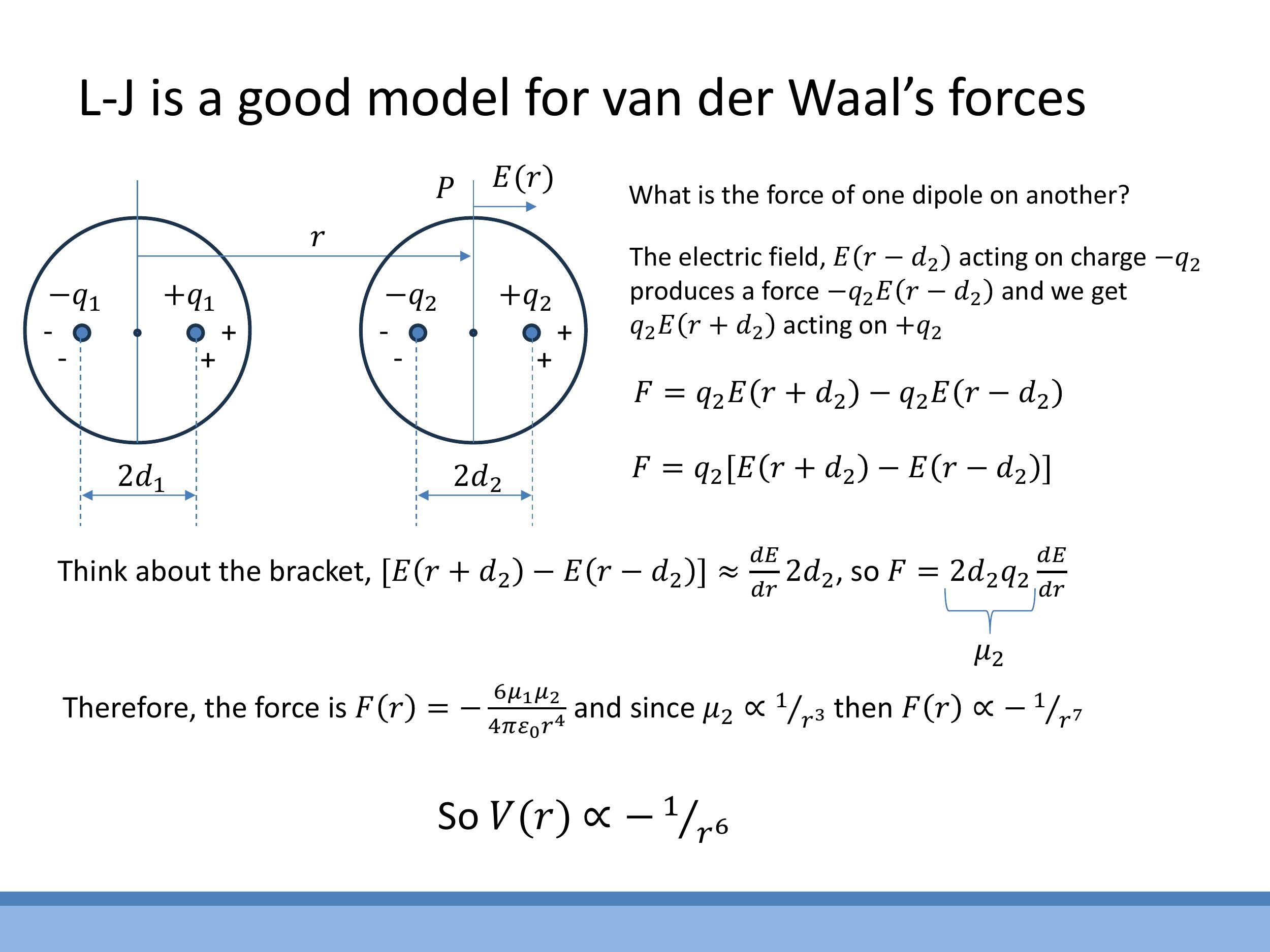
9) Appendix: Why van der Waals attraction scales as 1/r⁶ in V(r)
The attractive component of the van der Waals force, which leads to the $1/r^6$ dependence in the potential energy, can be understood through a derivation involving induced dipoles.
Side Note: This material is supplementary and won't be examined, but provides useful context.
The electric field $E(r)$ produced by a temporary dipole in one atom falls off as $1/r^3$ along its axis, which can be shown using a binomial approximation. This field then induces a dipole moment $\mu_2$ in a neighbouring neutral atom, with $\mu_2 \propto E \propto 1/r^3$. The force exerted on this induced dipole depends on the gradient of the electric field, $\frac{dE}{dr}$, which scales as $1/r^4$. Therefore, the attractive force $F$ between the two atoms is proportional to $\mu_2 \cdot \frac{dE}{dr}$, leading to an $F \propto (1/r^3)(1/r^4) = 1/r^7$ dependence. Integrating this force to obtain the potential energy, $V(r) = -\int F(r) \, dr $, results in an attractive potential that scales as $ -1/r^6$.
Key takeaways
Stable matter necessitates a balance between a very steep short-range repulsive interaction, primarily governed by the Pauli exclusion principle, and a weaker, longer-range attractive force, such as van der Waals interactions. The electromagnetic force is the dominant force responsible for interatomic bonding, overpowering gravity by approximately 32 orders of magnitude at Ångström-scale separations.
The states of matter-gas, liquid, and solid-are best distinguished by their physical properties of compressibility, rigidity, and viscosity, rather than simplified rules regarding density or regular atomic patterns, which often have exceptions. A tractable model for interatomic forces is given by $F(r) = A/r^m - B/r^n$, where $m > n$ ensures the dominance of repulsion at short distances, and equilibrium occurs at $F(a_0) = 0$.
Near equilibrium, atoms behave analogously to masses on springs, where the slope of the force-distance curve provides an effective spring constant. Integrating this force model yields the Lennard-Jones 12-6 potential, where $\varepsilon$ quantifies the bond strength (well depth), and $a$ defines a size scale where the potential energy is zero. The liquefaction of noble gases and the increasing boiling points down the noble gas group are directly explained by the strengthening of van der Waals forces originating from induced dipoles.
## Lecture 2: Interatomic Forces (part 1)
### 0) Orientation and learning outcomes
The "Properties of Matter" (PoM) course transitions from single-particle mechanics to the behaviour of many-body systems, exploring why solids, liquids, and gases exist and how their properties arise from atomic interactions. This lecture sets the foundational atomic picture, which will later be connected to classical thermodynamics.

Students are expected to describe solids, liquids, and gases using physical properties such as compressibility, rigidity, and viscosity. They should understand the major interatomic forces, recognise different bond types and their typical energies, and be able to sketch qualitative force-distance curves to identify equilibrium separation. Furthermore, students will learn to derive the Lennard-Jones potential from a two-term force model and use similar potentials to determine equilibrium distances and interpret key parameters like $\varepsilon$ (well depth) and $a$ (distance at $V=0$).
> *Side Note:* Any material marked "Appendix" throughout this course is supplementary, intended for interest only, and will not be assessed in examinations.
### 1) Why both attraction and repulsion must exist
The existence of condensed matter, such as solids and liquids, in everyday life unequivocally demonstrates the presence of attractive forces between atoms. Without such attraction, matter would not coalesce into stable structures. Conversely, if only attractive forces existed, all matter would collapse into an infinitely dense state. The stable, finite separations observed between atoms imply a necessary counteracting short-range repulsive force.
The dominant mechanism for this strong, short-range repulsion is the Pauli exclusion principle. This quantum mechanical rule dictates that no two electrons can occupy the same quantum state, meaning their wavefunctions cannot significantly overlap. As atoms approach each other, the overlap of electron clouds leads to a steep increase in energy, manifesting as a powerful repulsive force. While Coulombic repulsion between positively charged nuclei also exists, the Pauli exclusion principle provides the primary short-range repulsive barrier at typical interatomic distances.
This dynamic can be intuitively understood through a demonstration involving "inverter magnets." A central magnet experiences a weak, long-range attraction to surrounding magnets, creating a stable equilibrium point. However, if forced too close, a strong short-range repulsion rapidly pushes it away. If displaced from its equilibrium, the magnet "snaps back" to a stable separation, illustrating the balance between attractive and repulsive forces that establishes a stable "sweet spot" for interatomic distances.
### 2) States of matter: beyond the GCSE table
Traditional definitions of states of matter often rely on simplified rules that have significant exceptions. For instance, while solids are generally denser than liquids, and liquids denser than gases, water is a notable exception where ice (solid) is less dense than liquid water. Similarly, elements like silicon and germanium also exhibit this inverse density relationship. The notion that solids possess a "regular pattern" is also not universally true, as amorphous solids like glass, as well as polymers, lack long-range crystalline order.


A more physically robust characterisation of matter states focuses on properties such as compressibility, rigidity, and viscosity, alongside the microscopic motion of particles.
* **Gas:** Highly compressible, with low rigidity and viscosity. Particles are widely separated and exhibit fast, random translational motion. For example, nitrogen molecules in air at $20\,^\circ\text{C}$ have a root mean square speed of approximately $500\,\text{m s}^{-1}$.
* **Liquid:** Characterised by low compressibility and an absence of rigidity, but with viscosity typically about 100 times greater than that of a gas. Molecules are closely packed but can still move past one another, exhibiting a mixture of translational and vibrational motion.
* **Solid:** Rigid and possesses low compressibility, similar to liquids. Atoms in a solid primarily vibrate about fixed positions, with negligible translational motion.
While this course focuses on solid, liquid, and gas phases, it is worth noting that the vast majority of ordinary matter in the universe exists as plasma, a state where atoms are ionised.
### 3) Which fundamental force binds atoms? Range and scale
The four fundamental forces of nature are the strong nuclear force ($\sim 10^{-15}\,\text{m}$ range), the weak nuclear force ($\sim 10^{-18}\,\text{m}$ range), the electromagnetic force (infinite range), and gravity (infinite range). Given that typical interatomic separations are approximately $10^{-10}\,\text{m}$ (one Ångström, $1\,\text{Å}$), the strong and weak nuclear forces are too short-ranged to be relevant for atomic binding.
> **⚠️ Exam Alert!** The lecturer explicitly stated: "It's useful this number because it's going to help you whenever you face questions on the multiple choice exam in December, which will definitely ask you about this kind of thing or longer questions in the summer exam... if you've got an idea already of what a sensible distance is, you'll know whether you're on the right track or not." Students should remember that atomic distances are typically of the order of $1\,\text{Å}$ or $10^{-10}\,\text{m}$.
To determine the relative importance of gravity versus electromagnetism for interatomic bonding, a quantitative comparison of their potential energies can be performed. Consider two hypothetical atoms, each with a mass of $100\,\text{u}$ (atomic mass units) and charges of $\pm e$ (elementary charge), separated by a distance $r = 1.5\,\text{Å} = 1.5 \times 10^{-10}\,\text{m}$.
The gravitational potential energy $U_g$ is given by:
$$ U_g = -\frac{G m_1 m_2}{r} $$
The electrostatic potential energy $U_e$ is given by:
$$ U_e = \frac{q_1 q_2}{4\pi\varepsilon_0 r} $$
Calculating these values and converting to electron volts (eV) reveals $U_g \approx 7.65 \times 10^{-32}\,\text{eV}$ and $U_e \approx 9.6\,\text{eV}$. This demonstrates that the electromagnetic force is approximately $10^{32}$ times stronger than gravity at these scales, rendering gravity entirely negligible for interatomic bonding. Therefore, the electromagnetic force is the sole fundamental force responsible for holding atoms together.
### 4) Bond types as manifestations of electrostatics
All interatomic bonding originates from electrostatic interactions. These can be categorised into strong and weak bonds based on their energy scale.

Strong bonds, typically measured in electron volts, include:
* **Ionic bonds:** Formed by the complete transfer of electrons from one atom to another, creating positively and negatively charged ions (cations and anions) that are held together by strong Coulombic attraction, as seen in compounds like sodium chloride ($\text{NaCl}$).
* **Covalent bonds:** Involve the sharing of electron pairs between atoms, leading to strong, directional bonds.
* **Metallic bonds:** Consist of a lattice of positive ion cores immersed in a "sea" of delocalised valence electrons, resulting in strong, non-directional bonding.
Weaker, polar interactions include:
* **Hydrogen bonds:** Occur between molecules containing highly electronegative atoms (like oxygen or nitrogen) bonded to hydrogen, creating permanent dipoles that attract each other.
* **Van der Waals forces (London dispersion forces):** Arise from instantaneous fluctuations in electron clouds, creating temporary dipoles that induce dipoles in neighbouring atoms, leading to weak, transient attractions.
### 5) Van der Waals forces and noble gases
Noble gases, such as helium, neon, and argon, present a puzzle because they are chemically inert with fully occupied electron shells, yet they can be liquefied by cooling. This phenomenon cannot be explained by strong ionic, covalent, or metallic bonding.
The mechanism responsible is the London dispersion force, a type of van der Waals interaction. Due to the constant motion of electrons, an instantaneous, temporary fluctuation in the electron cloud of one atom can create a transient electric dipole. This temporary dipole then induces a corresponding dipole in a nearby neutral atom, resulting in a weak, short-lived attractive force between the two atoms. This transient nature is key to their weakness.

Empirical evidence supports this mechanism: the boiling points of noble gases increase progressively down the group (from helium to xenon). This trend is consistent with the increasing number of electrons and greater polarisability of larger atoms, which allows for stronger instantaneous and induced dipoles, leading to more significant van der Waals attractions and thus higher temperatures required for liquefaction.
### 6) A simple interatomic force model: two-term power law
A simple yet effective model for the net interatomic force $F(r)$ between two atoms at separation $r$ combines a short-range repulsive term and a longer-range attractive term. This is often expressed as:
$$ F(r) = \frac{A}{r^m} - \frac{B}{r^n} $$
Here, the first term, $A/r^m$, represents the repulsive force, while the second term, $-B/r^n$, represents the attractive force. The condition $m > n$ is crucial, as it ensures the repulsive force is much steeper and dominates at very short distances, preventing atomic collapse, while the attractive force has a longer range. Both forces tend to zero as $r \to \infty$.
The equilibrium separation distance, $a_0$, is defined as the point where the net force is zero, i.e., $F(a_0) = 0$. By setting $F(a_0) = 0$, a relationship between the coefficients $A$ and $B$ can be derived: $B = A a_0^{n-m}$. Substituting this back into the force equation allows $F(r)$ to be expressed in terms of $a_0$, which represents the stable "sweet spot" spacing in condensed phases. For van der Waals systems, typical exponents are $m \approx 13$ for the repulsive term and $n \approx 7$ for the attractive term. This model captures the essential qualitative features of interatomic interactions: a very steep repulsive wall at short distances (due to Pauli exclusion) and a gentler, longer-ranged attraction (e.g., van der Waals forces).

### 7) Near equilibrium: atoms behave like masses on springs (SHM)
For small displacements, $\delta r$, from the equilibrium separation $a_0$, the interatomic force curve can be approximated as linear. This linearisation is analogous to Hooke's Law for a spring. The change in force, $\delta F$, for a small displacement $\delta r$ is given by the gradient of the force curve at equilibrium:
$$ \delta F = \left.\frac{dF}{dr}\right|_{r=a_0} \cdot \delta r $$
This expression can be directly compared to Hooke's Law, $F = -k x$, where the effective spring constant $k_\text{eff}$ is defined as the negative of the gradient of the force curve at equilibrium: $k_\text{eff} = -\left.\frac{dF}{dr}\right|_{r=a_0}$. This implies that for small perturbations, atoms behave as if connected by springs, exerting a restoring force that brings them back to $a_0$.

This "atoms-as-springs" model is fundamental to understanding phenomena like thermal conductivity in non-metals. In such materials, heat is primarily transported by lattice vibrations, known as phonons, which are quantised wave packets of vibrational energy propagating through the interconnected atomic network.
### 8) From force to potential: arriving at Lennard-Jones
The interatomic potential energy $V(r)$ is related to the force $F(r)$ by the negative integral of the force with respect to distance, with the convention that the potential energy is zero at infinite separation, $V(\infty) = 0$:
$$ V(r) = -\int F(r)\,dr $$
Applying this integration to the two-term force model with van der Waals-like exponents ($m=13$ and $n=7$) yields the Lennard-Jones 12-6 potential:
$$ V(r) = 4\varepsilon \left[ \left(\frac{a}{r}\right)^{12} - \left(\frac{a}{r}\right)^6 \right] $$

In this widely used form, $\varepsilon$ represents the depth of the potential well, which corresponds to the energy required to separate the two atoms from their bound state (i.e., the bond strength). The parameter $a$ is the distance at which the potential energy $V(r)$ is zero, often used as an approximate atomic size or scale parameter in models. The minimum of this potential, corresponding to the equilibrium separation $r_\text{eq}$ (or $a_0$ in the force model), occurs at $r_\text{eq} = 2^{1/6}a$, which is slightly greater than $a$. The potential curve graphically illustrates a steep repulsive wall at short distances, a minimum at $r_\text{eq}$, and a gradual approach to zero from below as $r$ increases.
### 9) Appendix: Why van der Waals attraction scales as 1/r⁶ in V(r)
The attractive component of the van der Waals force, which leads to the $1/r^6$ dependence in the potential energy, can be understood through a derivation involving induced dipoles.
> *Side Note:* This material is supplementary and won't be examined, but provides useful context.
The electric field $E(r)$ produced by a temporary dipole in one atom falls off as $1/r^3$ along its axis, which can be shown using a binomial approximation. This field then induces a dipole moment $\mu_2$ in a neighbouring neutral atom, with $\mu_2 \propto E \propto 1/r^3$. The force exerted on this induced dipole depends on the gradient of the electric field, $\frac{dE}{dr}$, which scales as $1/r^4$. Therefore, the attractive force $F$ between the two atoms is proportional to $\mu_2 \cdot \frac{dE}{dr}$, leading to an $F \propto (1/r^3)(1/r^4) = 1/r^7$ dependence. Integrating this force to obtain the potential energy, $V(r) = -\int F(r)\,dr$, results in an attractive potential that scales as $-1/r^6$.


## Key takeaways
Stable matter necessitates a balance between a very steep short-range repulsive interaction, primarily governed by the Pauli exclusion principle, and a weaker, longer-range attractive force, such as van der Waals interactions. The electromagnetic force is the dominant force responsible for interatomic bonding, overpowering gravity by approximately 32 orders of magnitude at Ångström-scale separations.
The states of matter-gas, liquid, and solid-are best distinguished by their physical properties of compressibility, rigidity, and viscosity, rather than simplified rules regarding density or regular atomic patterns, which often have exceptions. A tractable model for interatomic forces is given by $F(r) = A/r^m - B/r^n$, where $m > n$ ensures the dominance of repulsion at short distances, and equilibrium occurs at $F(a_0) = 0$.
Near equilibrium, atoms behave analogously to masses on springs, where the slope of the force-distance curve provides an effective spring constant. Integrating this force model yields the Lennard-Jones 12-6 potential, where $\varepsilon$ quantifies the bond strength (well depth), and $a$ defines a size scale where the potential energy is zero. The liquefaction of noble gases and the increasing boiling points down the noble gas group are directly explained by the strengthening of van der Waals forces originating from induced dipoles.









