Lecture 5: Thermal Energy of Gases (part 2) and the Zeroth Law
0) Orientation, live demo, and quick recap
The lecture began with a live demonstration of the elastocaloric effect using an elastic band and a thermal imaging camera. When the band was stretched rapidly, its temperature visibly increased, appearing yellow on the thermal camera. If held in this stretched state, it would gradually cool back to room temperature. Conversely, a sudden release of the stretched band caused its temperature to decrease, appearing darker on the camera. Students were encouraged to feel these subtle temperature changes on their lips, which are sensitive enough to detect them. This demonstration served as an intuitive introduction to the relationships between work, heat, and internal energy, with a full microscopic explanation deferred until later in the course.
This lecture aims to complete the microscopic derivation that links the motion of gas particles to temperature. Following this, we'll introduce the Zeroth Law of Thermodynamics and the precise language used in thermodynamics. We'll then establish temperature on an absolute (Kelvin) scale and explore how it's measured, concluding with a glimpse into the microscopic, statistical meaning of temperature through the Boltzmann factor.
As a quick recap, our derivations are based on the ideal gas model, which assumes a large number of identical, non-interacting, point-like particles. These particles undergo perfectly elastic collisions with each other and the container walls, and their motion is entirely random. The very word "gas" originates from the Greek word "chaos," aptly reflecting this random molecular motion.
1) From wall impacts to pressure: finishing the kinetic-theory link
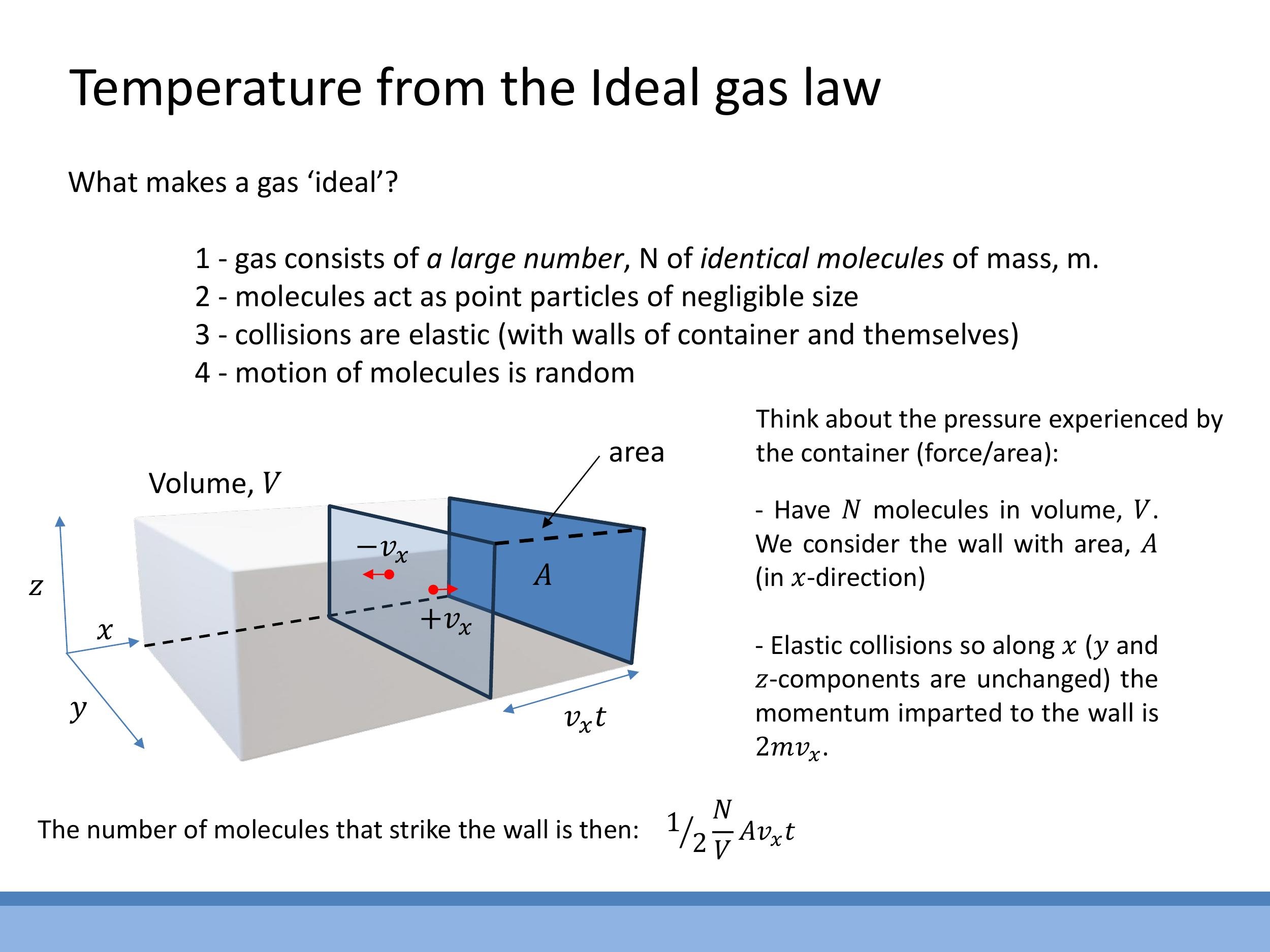
The pressure exerted by a gas arises from the continuous transfer of momentum when molecules collide elastically with the walls of their container. To quantify this, consider a single gas molecule of mass $m$ moving with velocity $v_x$ towards a wall. Upon an elastic collision, its momentum changes from $m v_x$ to $-m v_x$, meaning the momentum imparted to the wall is $2m v_x$.
To determine the total force on a wall of area $A$ over a time $t$, we need to count the number of such collisions. If there are $N$ molecules in a volume $V$, the number density is $N/V$. On average, half of these molecules will be moving towards any given wall. The number of molecules striking area $A$ in time $t$ is therefore given by $\frac{1}{2} \frac{N}{V} A v_x t$.
Multiplying the number of hits by the momentum change per hit gives the total impulse (force $\times$ time):
$$
\text{Total Impulse} = \left( \frac{1}{2} \frac{N}{V} A v_x t \right) \times (2 m v_x) = \frac{N}{V} A m v_x^2 t
$$
Dividing this total impulse by time $t$ yields the force on the wall:
$$
F = \frac{N}{V} A m v_x^2
$$
Finally, dividing the force by the area $A$ gives the pressure $P$:
$$
P = \frac{N}{V} m v_x^2
$$
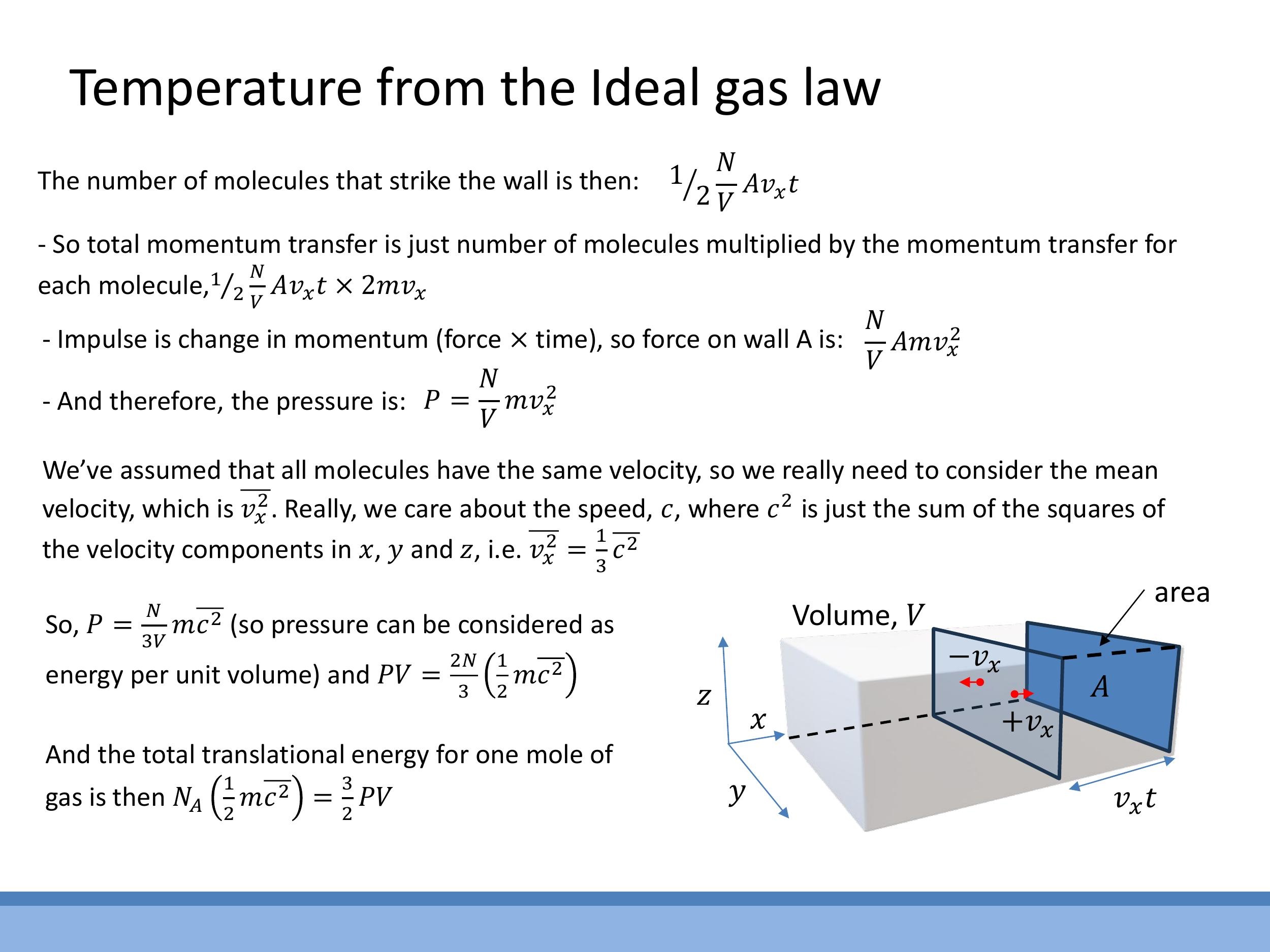
This expression, however, assumes all molecules move with the same velocity in the $x$ -direction. For a more realistic model of gas, we must consider random, three-dimensional motion. In such a scenario, the mean square velocity components are equal, so $\overline{v_x^2} = \overline{v_y^2} = \overline{v_z^2}$. The mean square speed $\overline{c^2}$ is the sum of these components, so $\overline{c^2} = \overline{v_x^2} + \overline{v_y^2} + \overline{v_z^2} = 3\overline{v_x^2}$. This leads to the relationship $\overline{v_x^2} = \frac{1}{3} \overline{c^2}$.
Substituting this into our pressure equation, we get:
$$
P = \frac{N}{3V} m \overline{c^2}
$$
This equation shows that pressure can be interpreted as an energy density. By rearranging, we can express the product $PV$ in terms of the average kinetic energy of the particles:
$$
PV = \frac{2N}{3} \left( \frac{1}{2} m \overline{c^2} \right)
$$
This expression connects the macroscopic properties of pressure and volume to the microscopic kinetic energy of the gas molecules.
2) Identifying temperature with average kinetic energy
The ideal gas law, $PV = nRT$, serves as a fundamental equation of state, linking the macroscopic state variables of pressure ($P$), volume ($V$), and temperature ($T$) for $n$ moles of an ideal gas, where $R$ is the ideal gas constant.
By equating this empirical equation of state with the kinetic theory derivation for $PV$ (for one mole, where $n=1$ and $N=N_A$ is Avogadro's number), we establish a crucial link:
$$
RT = \frac{2N_A}{3} \left( \frac{1}{2} m \overline{c^2} \right)
$$
To simplify this expression, we use Boltzmann's constant $k$, which is defined as $k = R/N_A$. Substituting this into the equation allows us to express the average translational kinetic energy of a single particle directly in terms of temperature:
$$
\frac{1}{2} m \overline{c^2} = \frac{3}{2} kT
$$
This fundamental result tells us that the average translational kinetic energy of a gas particle is directly proportional to the absolute temperature of the gas. This also implies that the average speed of a gas particle can be expressed as $\overline{c} = \sqrt{\frac{3kT}{m}}$.
In essence, this equation reveals that temperature is a direct measure of the average translational kinetic energy per particle in an ideal gas. The higher the temperature, the faster, on average, the particles are moving.
3) Equipartition and degrees of freedom: “½ kT per way to move”
The factor of $\frac{3}{2}$ in the expression $\frac{1}{2} m \overline{c^2} = \frac{3}{2} kT$ arises from the concept of degrees of freedom. For a monatomic ideal gas, such as helium, particles can only move translationally in three independent directions: $x$, $y$, and $z$. These are considered its three translational degrees of freedom.
The equipartition theorem states that, for a system in thermal equilibrium, each quadratic degree of freedom contributes an average energy of $\frac{1}{2} kT$. For a monatomic gas, with its three translational degrees of freedom, the total average translational energy is therefore $3 \times \frac{1}{2} kT = \frac{3}{2} kT$, which perfectly matches our derived result.
For more complex molecules, such as diatomic gases like nitrogen ($N_2$), additional degrees of freedom become relevant. Besides the three translational modes, diatomic molecules can also rotate about two axes perpendicular to their bond, adding two rotational degrees of freedom. At even higher temperatures, the atoms within the molecule can vibrate, contributing two more degrees of freedom (one for kinetic energy and one for potential energy of the vibration). Temperature effectively "unlocks" these extra modes as their characteristic energy spacings become thermally accessible, allowing them to absorb and store additional energy.
4) Building the thermodynamics vocabulary and the Zeroth Law
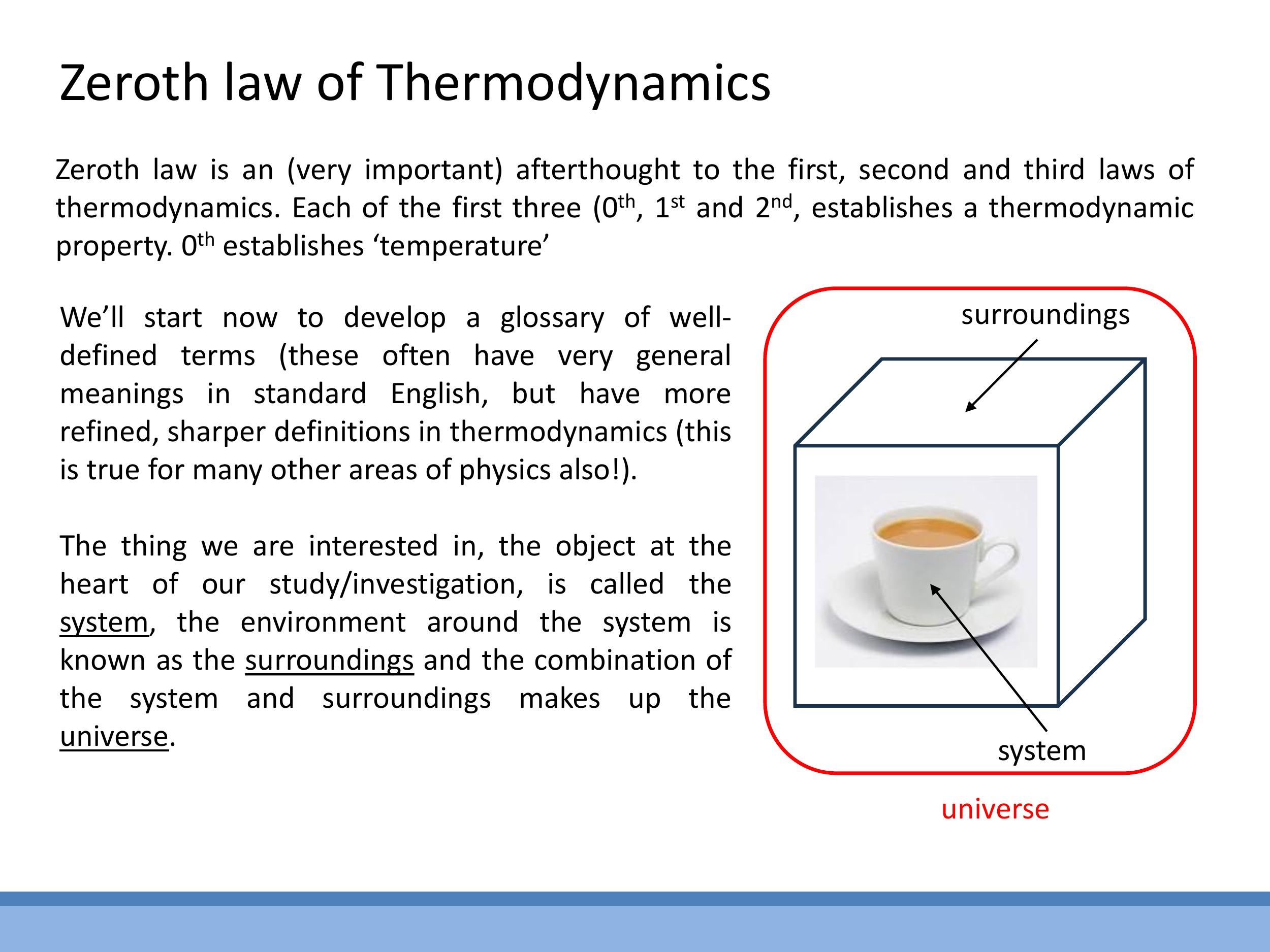
To discuss thermodynamics precisely, it's essential to establish a clear vocabulary. We define the system as the specific object or region of interest (for example, a cup of hot tea). The surroundings encompass everything in the immediate vicinity that interacts with the system. Together, the system and its surroundings constitute the universe in a thermodynamic context.
Systems can be classified based on what they exchange with their surroundings:
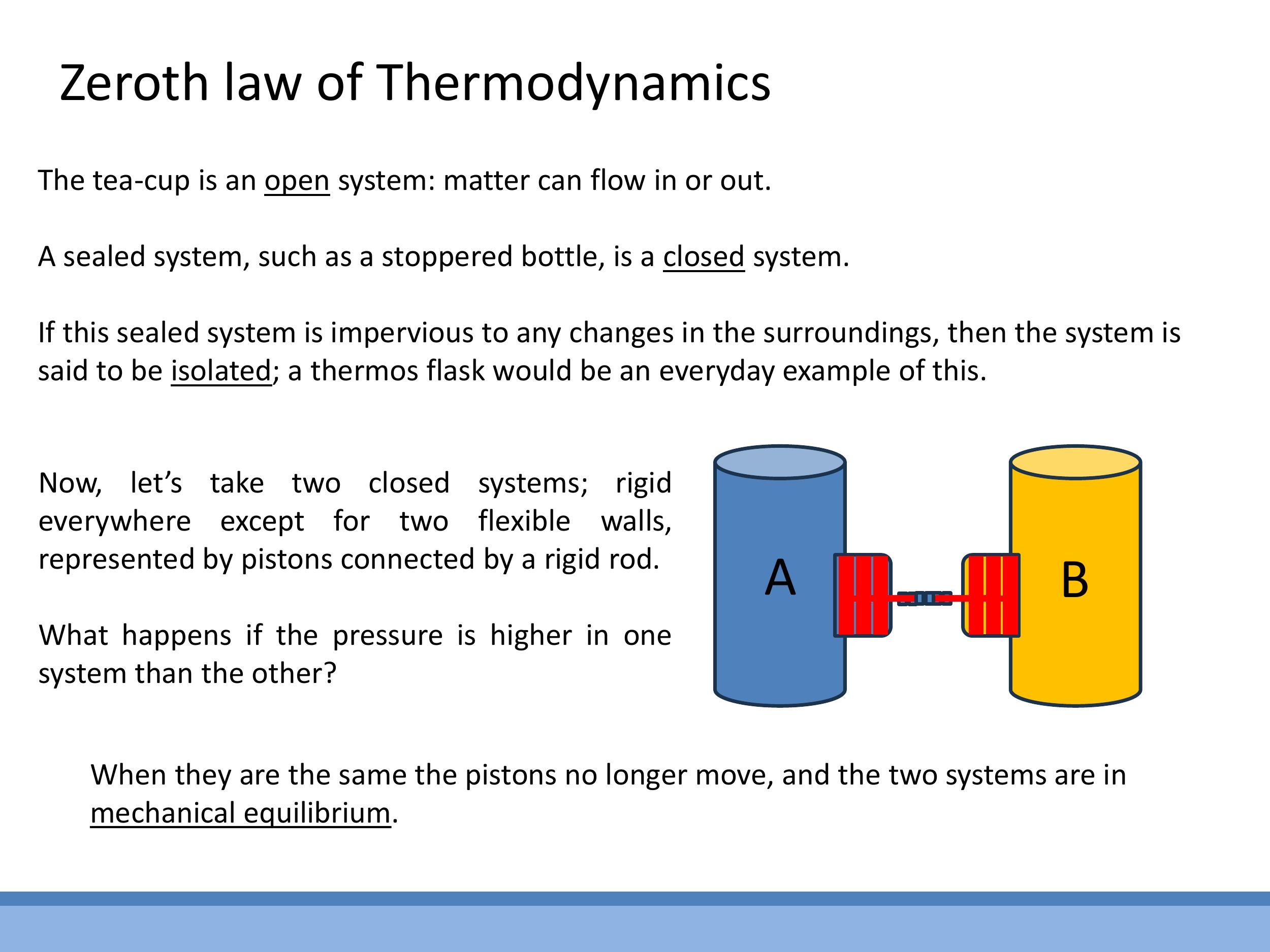
- An open system exchanges both matter and energy (e.g., an open cup of tea, where water vapour can escape).
- A closed system exchanges energy but not matter (e.g., a stoppered bottle).
- An isolated system exchanges neither matter nor energy (e.g., an ideal thermos flask).
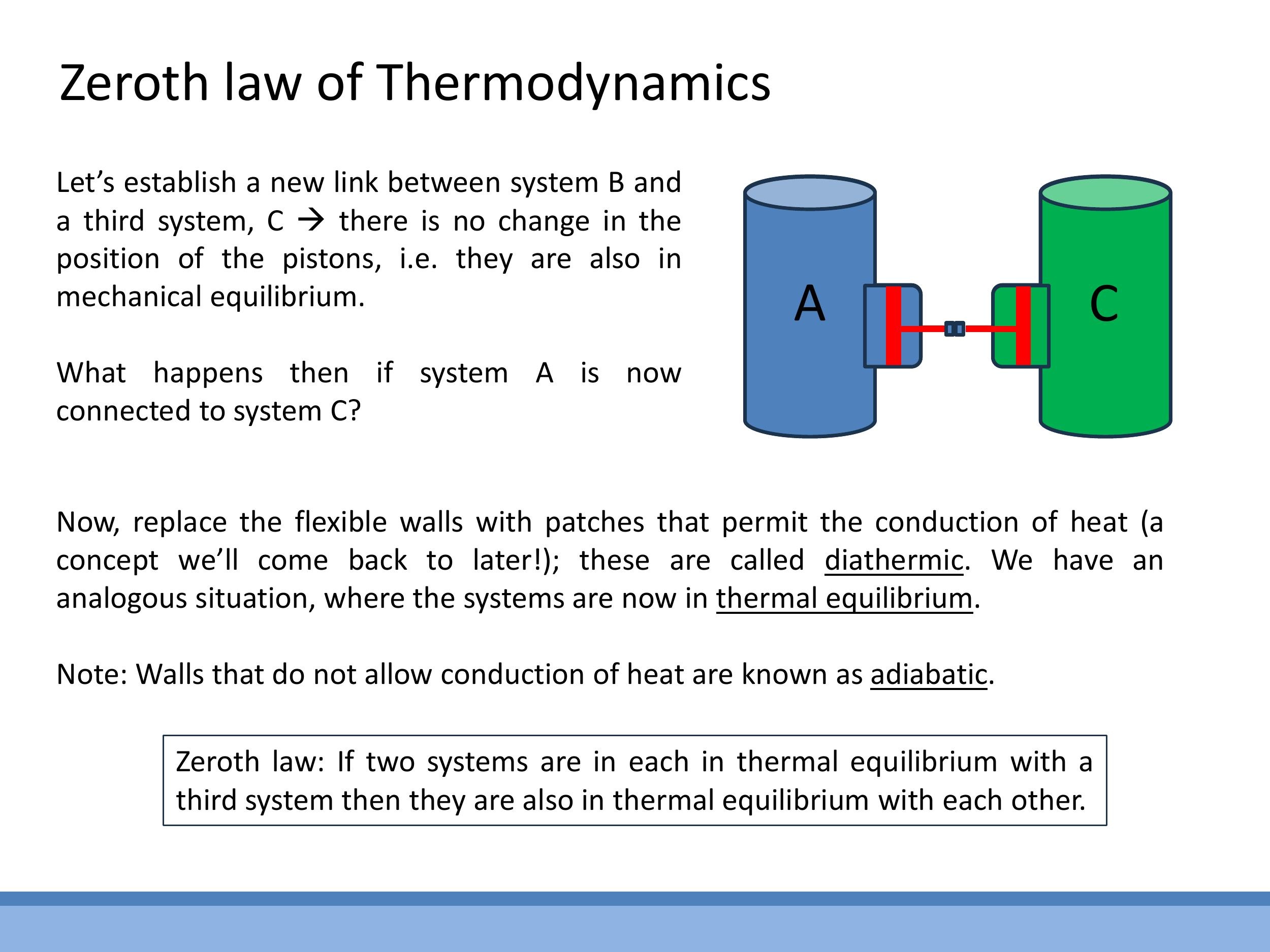
The concept of equilibrium is central to thermodynamics. Consider an analogy for mechanical equilibrium: if two pressurised vessels (A and B) are connected by a free-moving piston, and the piston remains stationary, it implies that the pressures in both vessels are equal, and they are in mechanical equilibrium. This property is transitive: if system A is in mechanical equilibrium with system B, and system B is in mechanical equilibrium with system C, then A must also be in mechanical equilibrium with C.
We can extend this idea to thermal equilibrium diathermic walls, which permit the conduction of heat, or adiabatic walls, which prevent it. If two systems are brought into thermal contact via diathermic walls and no net energy flows between them, they are in thermal equilibrium. This transitivity principle for thermal equilibrium is formalised by the Zeroth Law.
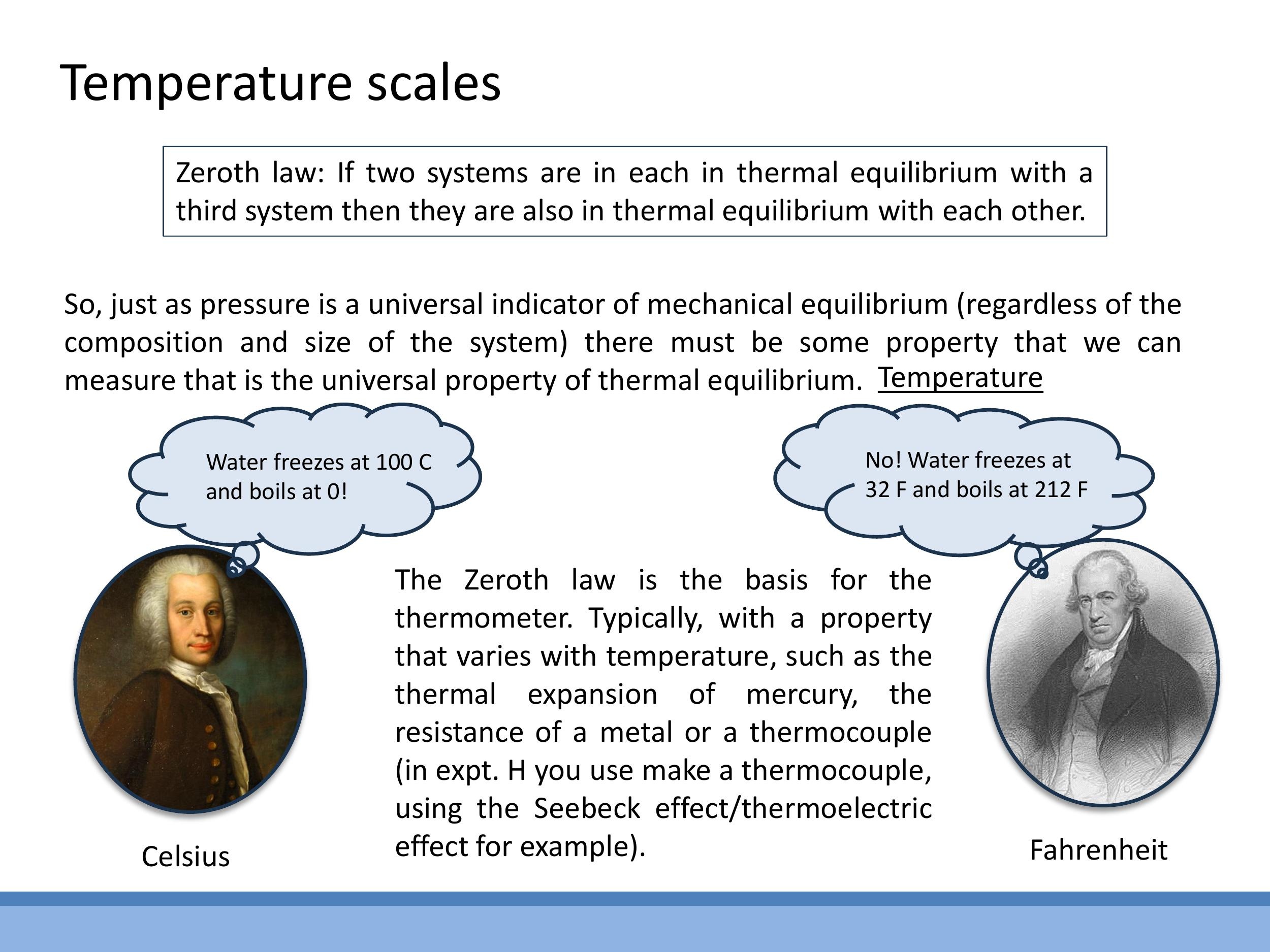
The Zeroth Law of Thermodynamics states: If two systems are each in thermal equilibrium with a third system, then they are also in thermal equilibrium with each other. This law is fundamental because it allows us to define and measure temperature. It justifies the use of a thermometer: if a thermometer (the third system) is in thermal equilibrium with one object, and then shows the same reading when in thermal equilibrium with a second object, we can conclude that the two objects have the same temperature, regardless of their composition.
5) Temperature scales and absolute temperature (Kelvin)
Historically, various temperature scales emerged. Celsius's original scale, for instance, was inverted compared to modern use, with $0 \, ^{\circ}\text{C} $ at boiling and $ 100 \, ^{\circ}\text{C}$ at freezing. Fahrenheit's scale was based on body temperature and a reproducible cold mixture. However, for scientific work, an absolute thermodynamic temperature scale is essential, where $T=0 \, \text{K}$ represents absolute zero, the theoretical lowest possible temperature. The Kelvin (K) scale is the standard SI absolute scale, sharing its step size with the Celsius scale. The Rankine scale is its Fahrenheit-based equivalent.
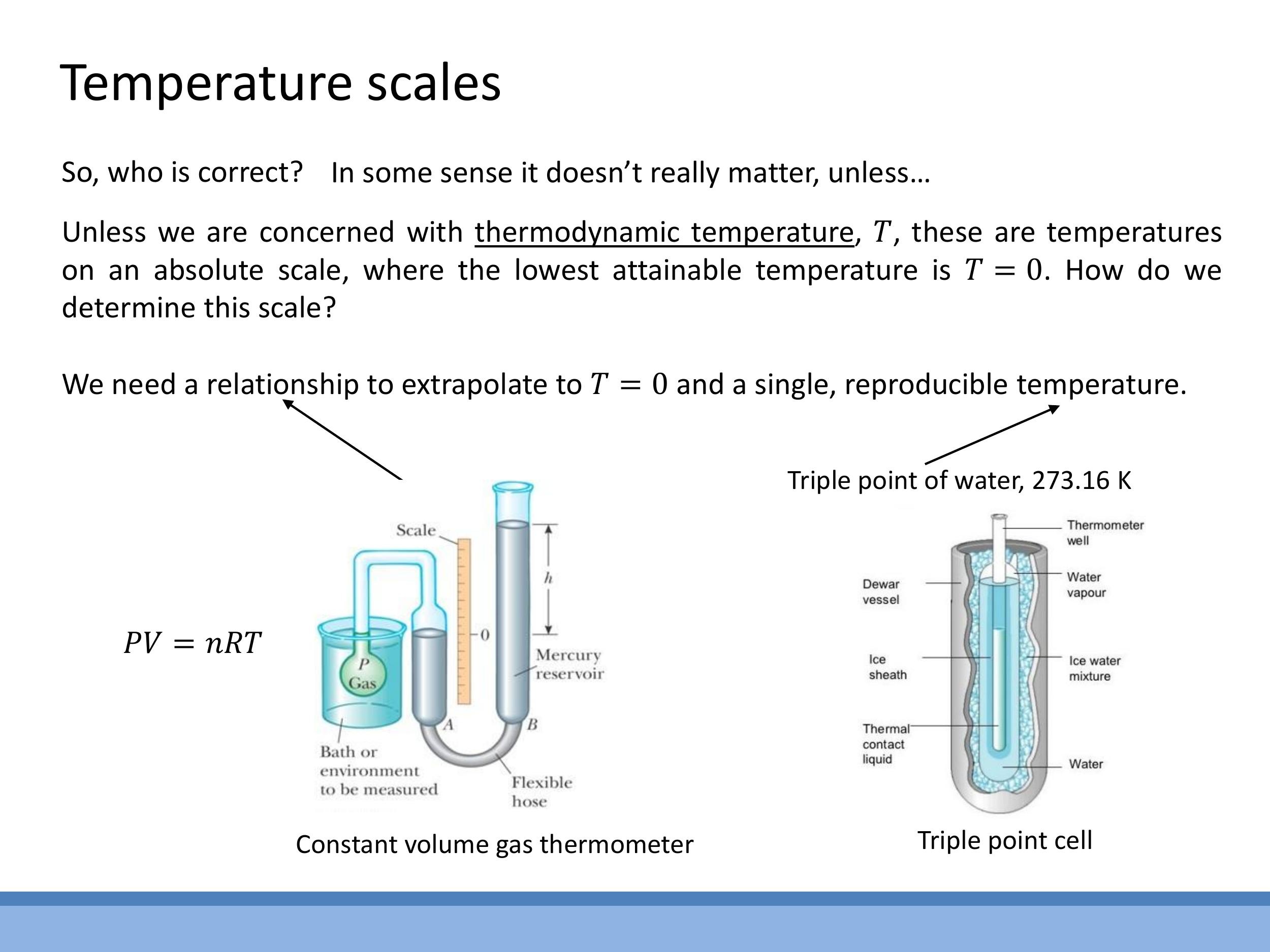
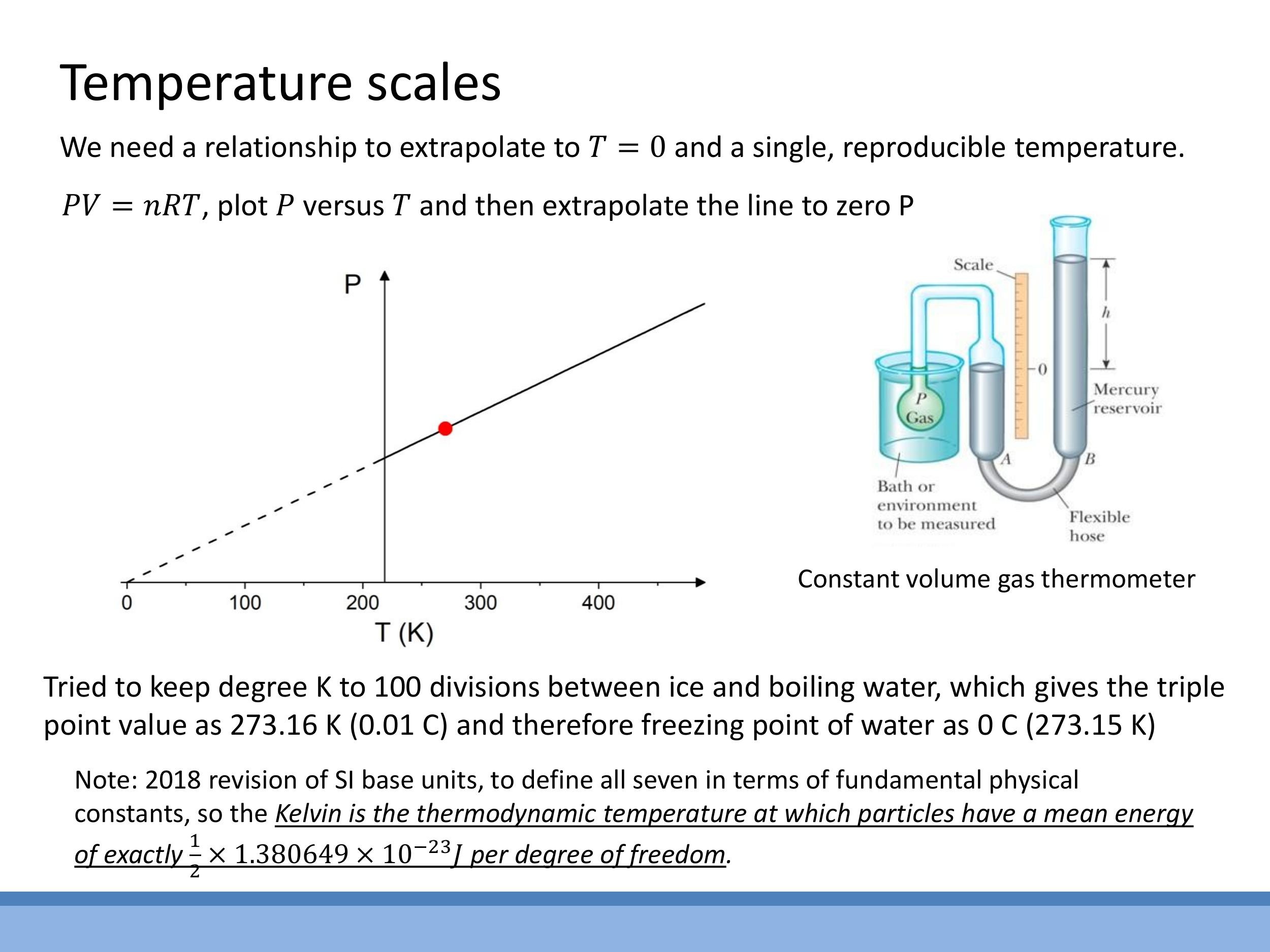
Establishing an absolute temperature scale requires a reproducible reference point and a method for extrapolation. Freezing and boiling points of water are unsuitable as they vary with pressure. Instead, the triple point of water is used, where solid ice, liquid water, and water vapour coexist in stable equilibrium. This unique point is defined as $273.16 \, \text{K} $ ($ 0.01 \, ^{\circ}\text{C}$).
An absolute scale can be measured using a constant-volume gas thermometer
The modern SI definition (2018) for the Kelvin scale ties temperature directly to energy. The Kelvin is now defined by fixing the numerical value of Boltzmann's constant $k$, meaning the Kelvin is the thermodynamic temperature at which particles have a mean energy of exactly $\frac{1}{2} \times 1.380649 \times 10^{-23} \, \text{J}$ per degree of freedom.
6) Microscopic temperature: Boltzmann factor and distributions
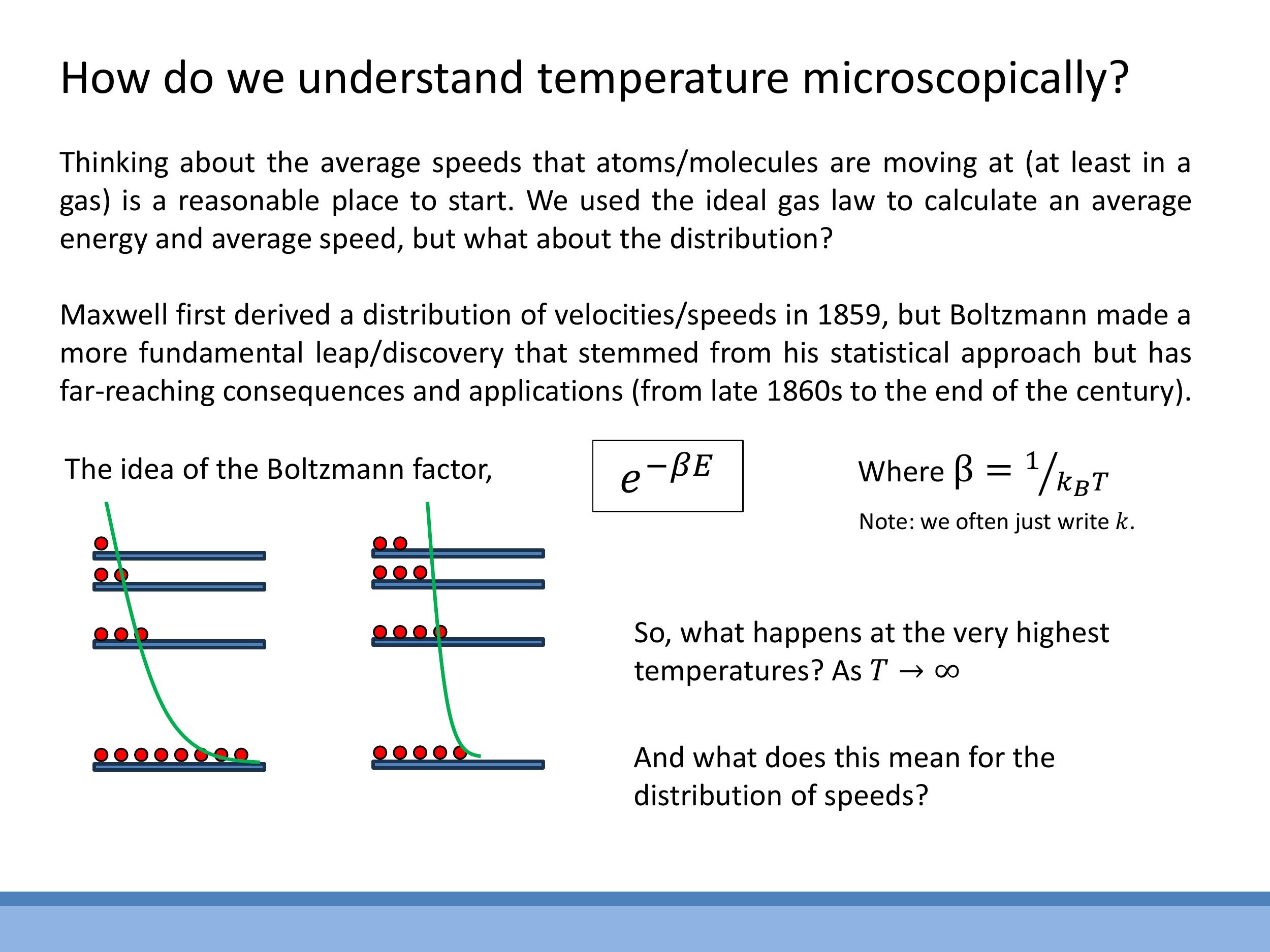
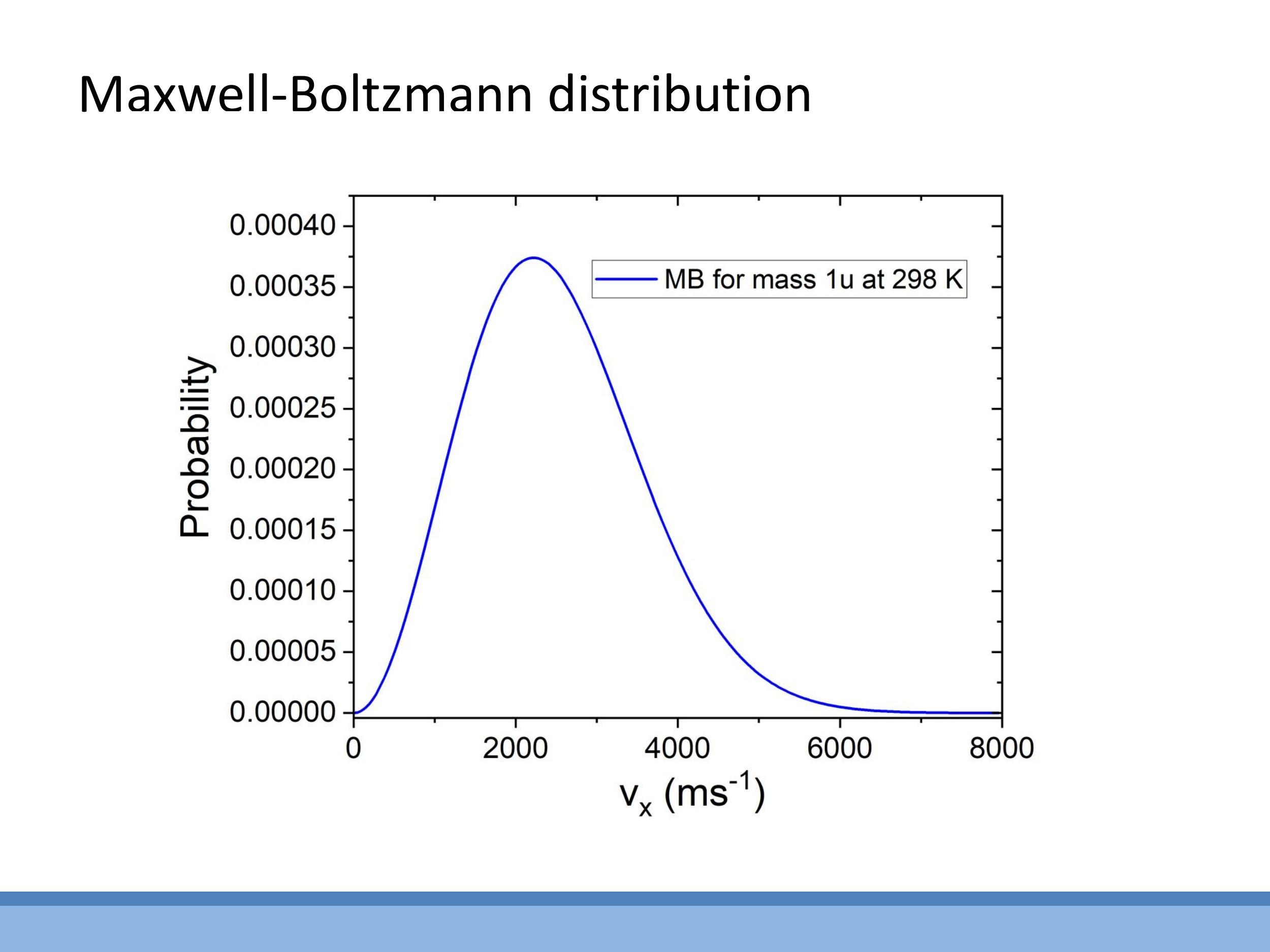
While the average kinetic energy $\frac{3}{2} kT$ provides a useful macroscopic measure of temperature, a deeper microscopic understanding involves considering the distribution of particle speeds. The Maxwell-Boltzmann distribution, for example, describes the spread of speeds and velocities in a gas at a given temperature, and its shape changes with temperature and particle mass.
At its core, this distribution is governed by the Boltzmann factor, $e^{-\beta E}$, where $\beta = 1/(kT)$. This factor quantifies the probability weight for a system or particle to be in a state with energy $E$ at a given temperature $T$. We can visualise this with an analogy of "balls on shelves," where the shelves represent quantised energy levels and the balls represent particles. At low temperatures, most particles occupy the lowest energy levels (bottom shelves). As temperature increases, the Boltzmann factor allows more particles to access and populate higher energy levels, leading to a broader distribution across the shelves.
This statistical approach leads to conceptual questions, such as what happens to these distributions as the temperature approaches infinity ($T \rightarrow \infty$, meaning $\beta \rightarrow 0$), which would cause the distributions to flatten, indicating an equal population across all energy states. Another intriguing question is what a "population inversion" (more particles in higher energy states than lower ones) would mean in this statistical language. These advanced implications will be explored in later discussions.
7) Bridge back to the demo and forward to next topics
The live demonstration of the elastic band heating upon rapid stretching and cooling upon sudden release provides a tangible illustration of how doing work can alter a system's internal energy and temperature. These observations directly set the stage for our upcoming discussions on heat, work, and the First Law of Thermodynamics. In subsequent lectures, we will delve deeper into the First and Second Laws, allowing us to revisit the elastic band demo with a more complete understanding of energy accounting.
Key takeaways
- Kinetic theory to temperature: Pressure arises from molecular momentum transfer, described by $PV = \frac{2N}{3} \left( \frac{1}{2} m \overline{c^2} \right)$. This leads to the fundamental relationship $\frac{1}{2} m \overline{c^2} = \frac{3}{2} kT$, showing that temperature is directly proportional to the average translational kinetic energy per particle. The average speed of particles is given by $\overline{c} = \sqrt{\frac{3kT}{m}}$.
- Equipartition Theorem: Each quadratic degree of freedom contributes an average energy of $\frac{1}{2} kT$ at thermal equilibrium. For monatomic gases, this means the total translational energy is $\frac{3}{2} kT$. More complex molecules can also access rotational and vibrational modes as temperature increases.
- Thermodynamics language: Key terms include system, surroundings, and universe. Systems can be open (exchanging matter and energy), closed (exchanging energy only), or isolated (exchanging neither). Walls can be diathermic (permitting heat flow) or adiabatic (preventing heat flow).
- Zeroth Law: This law states that if two systems are each in thermal equilibrium with a third, they are in thermal equilibrium with each other. It underpins the fundamental concept of temperature and the operation of thermometers.
- Absolute temperature: The Kelvin scale is the absolute thermodynamic temperature scale, with $T=0 \, \text{K} $ representing absolute zero. It is established using constant-volume gas thermometry and calibrated using the triple point of water ($ 273.16 \, \text{K} $). The modern SI definition of the Kelvin fixes Boltzmann's constant $ k$, directly linking temperature to energy.
- Microscopic view: Beyond simple averages, Maxwell-Boltzmann distributions describe the spread of molecular speeds. The Boltzmann factor, $e^{-\beta E}$ (where $\beta = 1/(kT)$), is central to understanding how temperature dictates the occupancy of different energy levels at a microscopic scale.
## Lecture 5: Thermal Energy of Gases (part 2) and the Zeroth Law
### 0) Orientation, live demo, and quick recap
The lecture began with a live demonstration of the elastocaloric effect using an elastic band and a thermal imaging camera. When the band was stretched rapidly, its temperature visibly increased, appearing yellow on the thermal camera. If held in this stretched state, it would gradually cool back to room temperature. Conversely, a sudden release of the stretched band caused its temperature to decrease, appearing darker on the camera. Students were encouraged to feel these subtle temperature changes on their lips, which are sensitive enough to detect them. This demonstration served as an intuitive introduction to the relationships between work, heat, and internal energy, with a full microscopic explanation deferred until later in the course.
This lecture aims to complete the microscopic derivation that links the motion of gas particles to temperature. Following this, we'll introduce the Zeroth Law of Thermodynamics and the precise language used in thermodynamics. We'll then establish temperature on an absolute (Kelvin) scale and explore how it's measured, concluding with a glimpse into the microscopic, statistical meaning of temperature through the Boltzmann factor.
As a quick recap, our derivations are based on the ideal gas model, which assumes a large number of identical, non-interacting, point-like particles. These particles undergo perfectly elastic collisions with each other and the container walls, and their motion is entirely random. The very word "gas" originates from the Greek word "chaos," aptly reflecting this random molecular motion.
### 1) From wall impacts to pressure: finishing the kinetic-theory link


The pressure exerted by a gas arises from the continuous transfer of momentum when molecules collide elastically with the walls of their container. To quantify this, consider a single gas molecule of mass $m$ moving with velocity $v_x$ towards a wall. Upon an elastic collision, its momentum changes from $m v_x$ to $-m v_x$, meaning the momentum imparted to the wall is $2m v_x$.
To determine the total force on a wall of area $A$ over a time $t$, we need to count the number of such collisions. If there are $N$ molecules in a volume $V$, the number density is $N/V$. On average, half of these molecules will be moving towards any given wall. The number of molecules striking area $A$ in time $t$ is therefore given by $\frac{1}{2} \frac{N}{V} A v_x t$.
Multiplying the number of hits by the momentum change per hit gives the total impulse (force $\times$ time):
$$ \text{Total Impulse} = \left( \frac{1}{2} \frac{N}{V} A v_x t \right) \times (2 m v_x) = \frac{N}{V} A m v_x^2 t $$
Dividing this total impulse by time $t$ yields the force on the wall:
$$ F = \frac{N}{V} A m v_x^2 $$
Finally, dividing the force by the area $A$ gives the pressure $P$:
$$ P = \frac{N}{V} m v_x^2 $$
This expression, however, assumes all molecules move with the same velocity in the $x$-direction. For a more realistic model of gas, we must consider random, three-dimensional motion. In such a scenario, the mean square velocity components are equal, so $\overline{v_x^2} = \overline{v_y^2} = \overline{v_z^2}$. The mean square speed $\overline{c^2}$ is the sum of these components, so $\overline{c^2} = \overline{v_x^2} + \overline{v_y^2} + \overline{v_z^2} = 3\overline{v_x^2}$. This leads to the relationship $\overline{v_x^2} = \frac{1}{3} \overline{c^2}$.
Substituting this into our pressure equation, we get:
$$ P = \frac{N}{3V} m \overline{c^2} $$
This equation shows that pressure can be interpreted as an energy density. By rearranging, we can express the product $PV$ in terms of the average kinetic energy of the particles:
$$ PV = \frac{2N}{3} \left( \frac{1}{2} m \overline{c^2} \right) $$
This expression connects the macroscopic properties of pressure and volume to the microscopic kinetic energy of the gas molecules.
### 2) Identifying temperature with average kinetic energy
The ideal gas law, $PV = nRT$, serves as a fundamental equation of state, linking the macroscopic state variables of pressure ($P$), volume ($V$), and temperature ($T$) for $n$ moles of an ideal gas, where $R$ is the ideal gas constant.
By equating this empirical equation of state with the kinetic theory derivation for $PV$ (for one mole, where $n=1$ and $N=N_A$ is Avogadro's number), we establish a crucial link:
$$ RT = \frac{2N_A}{3} \left( \frac{1}{2} m \overline{c^2} \right) $$
To simplify this expression, we use Boltzmann's constant $k$, which is defined as $k = R/N_A$. Substituting this into the equation allows us to express the average translational kinetic energy of a single particle directly in terms of temperature:
$$ \frac{1}{2} m \overline{c^2} = \frac{3}{2} kT $$
This fundamental result tells us that the average translational kinetic energy of a gas particle is directly proportional to the absolute temperature of the gas. This also implies that the average speed of a gas particle can be expressed as $\overline{c} = \sqrt{\frac{3kT}{m}}$.
In essence, this equation reveals that temperature is a direct measure of the average translational kinetic energy per particle in an ideal gas. The higher the temperature, the faster, on average, the particles are moving.
### 3) Equipartition and degrees of freedom: “½ kT per way to move”
The factor of $\frac{3}{2}$ in the expression $\frac{1}{2} m \overline{c^2} = \frac{3}{2} kT$ arises from the concept of degrees of freedom. For a monatomic ideal gas, such as helium, particles can only move translationally in three independent directions: $x$, $y$, and $z$. These are considered its three translational degrees of freedom.
The equipartition theorem states that, for a system in thermal equilibrium, each quadratic degree of freedom contributes an average energy of $\frac{1}{2} kT$. For a monatomic gas, with its three translational degrees of freedom, the total average translational energy is therefore $3 \times \frac{1}{2} kT = \frac{3}{2} kT$, which perfectly matches our derived result.
For more complex molecules, such as diatomic gases like nitrogen ($N_2$), additional degrees of freedom become relevant. Besides the three translational modes, diatomic molecules can also rotate about two axes perpendicular to their bond, adding two rotational degrees of freedom. At even higher temperatures, the atoms within the molecule can vibrate, contributing two more degrees of freedom (one for kinetic energy and one for potential energy of the vibration). Temperature effectively "unlocks" these extra modes as their characteristic energy spacings become thermally accessible, allowing them to absorb and store additional energy.
### 4) Building the thermodynamics vocabulary and the Zeroth Law




To discuss thermodynamics precisely, it's essential to establish a clear vocabulary. We define the **system** as the specific object or region of interest (for example, a cup of hot tea). The **surroundings** encompass everything in the immediate vicinity that interacts with the system. Together, the system and its surroundings constitute the **universe** in a thermodynamic context.
Systems can be classified based on what they exchange with their surroundings:
* An **open system** exchanges both matter and energy (e.g., an open cup of tea, where water vapour can escape).
* A **closed system** exchanges energy but not matter (e.g., a stoppered bottle).
* An **isolated system** exchanges neither matter nor energy (e.g., an ideal thermos flask).
The concept of equilibrium is central to thermodynamics. Consider an analogy for **mechanical equilibrium**: if two pressurised vessels (A and B) are connected by a free-moving piston, and the piston remains stationary, it implies that the pressures in both vessels are equal, and they are in mechanical equilibrium. This property is transitive: if system A is in mechanical equilibrium with system B, and system B is in mechanical equilibrium with system C, then A must also be in mechanical equilibrium with C.
We can extend this idea to **thermal equilibrium**. Instead of rigid, movable walls, imagine systems separated by **diathermic walls**, which permit the conduction of heat, or **adiabatic walls**, which prevent it. If two systems are brought into thermal contact via diathermic walls and no net energy flows between them, they are in thermal equilibrium. This transitivity principle for thermal equilibrium is formalised by the Zeroth Law.
The **Zeroth Law of Thermodynamics** states: If two systems are each in thermal equilibrium with a third system, then they are also in thermal equilibrium with each other. This law is fundamental because it allows us to define and measure temperature. It justifies the use of a thermometer: if a thermometer (the third system) is in thermal equilibrium with one object, and then shows the same reading when in thermal equilibrium with a second object, we can conclude that the two objects have the same temperature, regardless of their composition.
### 5) Temperature scales and absolute temperature (Kelvin)


Historically, various temperature scales emerged. Celsius's original scale, for instance, was inverted compared to modern use, with $0\,^{\circ}\text{C}$ at boiling and $100\,^{\circ}\text{C}$ at freezing. Fahrenheit's scale was based on body temperature and a reproducible cold mixture. However, for scientific work, an **absolute thermodynamic temperature** scale is essential, where $T=0\,\text{K}$ represents absolute zero, the theoretical lowest possible temperature. The **Kelvin (K)** scale is the standard SI absolute scale, sharing its step size with the Celsius scale. The **Rankine** scale is its Fahrenheit-based equivalent.
Establishing an absolute temperature scale requires a reproducible reference point and a method for extrapolation. Freezing and boiling points of water are unsuitable as they vary with pressure. Instead, the **triple point of water** is used, where solid ice, liquid water, and water vapour coexist in stable equilibrium. This unique point is defined as $273.16\,\text{K}$ ($0.01\,^{\circ}\text{C}$).
An absolute scale can be measured using a **constant-volume gas thermometer**. In this device, the pressure of a fixed amount of gas at constant volume is directly proportional to its absolute temperature ($P \propto T$). By measuring the pressure at the triple point of water and other temperatures, a linear relationship between $P$ and $T$ can be established. Extrapolating this line to zero pressure ($P \rightarrow 0$) allows us to precisely locate absolute zero ($T \rightarrow 0\,\text{K}$), as pressure intuitively vanishes when particle motion ceases.
The modern SI definition (2018) for the Kelvin scale ties temperature directly to energy. The Kelvin is now defined by fixing the numerical value of Boltzmann's constant $k$, meaning the Kelvin is the thermodynamic temperature at which particles have a mean energy of exactly $\frac{1}{2} \times 1.380649 \times 10^{-23}\,\text{J}$ per degree of freedom.
### 6) Microscopic temperature: Boltzmann factor and distributions


While the average kinetic energy $\frac{3}{2} kT$ provides a useful macroscopic measure of temperature, a deeper microscopic understanding involves considering the **distribution** of particle speeds. The Maxwell-Boltzmann distribution, for example, describes the spread of speeds and velocities in a gas at a given temperature, and its shape changes with temperature and particle mass.
At its core, this distribution is governed by the **Boltzmann factor**, $e^{-\beta E}$, where $\beta = 1/(kT)$. This factor quantifies the probability weight for a system or particle to be in a state with energy $E$ at a given temperature $T$. We can visualise this with an analogy of "balls on shelves," where the shelves represent quantised energy levels and the balls represent particles. At low temperatures, most particles occupy the lowest energy levels (bottom shelves). As temperature increases, the Boltzmann factor allows more particles to access and populate higher energy levels, leading to a broader distribution across the shelves.
This statistical approach leads to conceptual questions, such as what happens to these distributions as the temperature approaches infinity ($T \rightarrow \infty$, meaning $\beta \rightarrow 0$), which would cause the distributions to flatten, indicating an equal population across all energy states. Another intriguing question is what a "population inversion" (more particles in higher energy states than lower ones) would mean in this statistical language. These advanced implications will be explored in later discussions.
### 7) Bridge back to the demo and forward to next topics
The live demonstration of the elastic band heating upon rapid stretching and cooling upon sudden release provides a tangible illustration of how doing work can alter a system's internal energy and temperature. These observations directly set the stage for our upcoming discussions on heat, work, and the First Law of Thermodynamics. In subsequent lectures, we will delve deeper into the First and Second Laws, allowing us to revisit the elastic band demo with a more complete understanding of energy accounting.
## Key takeaways
* **Kinetic theory to temperature:** Pressure arises from molecular momentum transfer, described by $PV = \frac{2N}{3} \left( \frac{1}{2} m \overline{c^2} \right)$. This leads to the fundamental relationship $\frac{1}{2} m \overline{c^2} = \frac{3}{2} kT$, showing that temperature is directly proportional to the average translational kinetic energy per particle. The average speed of particles is given by $\overline{c} = \sqrt{\frac{3kT}{m}}$.
* **Equipartition Theorem:** Each quadratic degree of freedom contributes an average energy of $\frac{1}{2} kT$ at thermal equilibrium. For monatomic gases, this means the total translational energy is $\frac{3}{2} kT$. More complex molecules can also access rotational and vibrational modes as temperature increases.
* **Thermodynamics language:** Key terms include **system**, **surroundings**, and **universe**. Systems can be **open** (exchanging matter and energy), **closed** (exchanging energy only), or **isolated** (exchanging neither). Walls can be **diathermic** (permitting heat flow) or **adiabatic** (preventing heat flow).
* **Zeroth Law:** This law states that if two systems are each in thermal equilibrium with a third, they are in thermal equilibrium with each other. It underpins the fundamental concept of temperature and the operation of thermometers.
* **Absolute temperature:** The Kelvin scale is the absolute thermodynamic temperature scale, with $T=0\,\text{K}$ representing absolute zero. It is established using constant-volume gas thermometry and calibrated using the triple point of water ($273.16\,\text{K}$). The modern SI definition of the Kelvin fixes Boltzmann's constant $k$, directly linking temperature to energy.
* **Microscopic view:** Beyond simple averages, Maxwell-Boltzmann distributions describe the spread of molecular speeds. The **Boltzmann factor**, $e^{-\beta E}$ (where $\beta = 1/(kT)$), is central to understanding how temperature dictates the occupancy of different energy levels at a microscopic scale.









